![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
62 Cards in this Set
- Front
- Back
|
Lipid functions |
Energy source Membrane components Storage (triglycerides) Regulatory functions (white adipose) Thermogenesis (brown adipose) |
|
|
Why do we need to add fat to animal diets? |
1. Increase energy density of diet 2. Other benefits (improve diet and pellet characteristics, lubricate equipment, improve metabolic efficiency, etc) |
|
|
What happens to lipids in the abomasum during lipid digestion? (ruminant) |
Ca-salts of FAs dissociate under acidic pH |
|
|
What happens to lipids in the oral cavity? (monogastrics) |
Minimal digestion by lingual lipase |
|
|
What happens to lipids in the corpus and fundus during lipid digestion? (monogastrics) |
Mechanical emulsification Gastric lipase releases DAG and FA Intense mixing and liquefaction of fat globules to form lipid droplets |
|
|
What happens to lipids in the duodenum during lipid digestion? (ruminants) |
Emulsification of lipid droplets by bile and lipolysis by pancreatic lipase and colipase Rumen bile rich in taurocholic acids *~20% lipid absorption |
|
|
What happens to lipids in the duodenum during lipid digestion? (monogastrics) |
Emulsification of lipid droplets by bile and lipolysis by pancreatic lipase and colipase *~20% lipid absorption
(similar to ruminants!) |
|
|
What happens to lipids in the jejunum during lipid digestion? (both ruminants and monogastrics) |
Micelle formation by the combined action of bile, pancreatic lipase and colipase *60-70% lipid absorption (majority!) |
|
|
What happens to lipids in the ileum during lipid digestion? (both ruminants and monogastrics) |
Bile acid reabsorption by ASBT *10-15% lipid absorption |
|
|
Fate of lipids in the rumen: 1. Fatty Acids 2. Glycerolipids 3. Triglycerides |
1. Hydrolysis and biohydrogenation 2. Hydrolysis and biohydrogenation 3. Biohydrogenation or bypass |
|
|
Which fats bypass the rumen? |
Inert fats (protected against rumen digestion) |
|
|
Biohydrogenation: definition |
The addition of an H to solidify an unsaturated FA; make it more saturated |
|
|
The greater the number of double bonds, the __________ the degree of biohydrogenation |
Greater |
|
|
Hydrolysis occurs by the action of? Hydrolysis occurs very ______? Hydrolysis inhibited by? |
Bacterial and protozoal enzymes
Rapidly
Low pH and antibiotics |
|
|
Biohydrogenation is greater in what type of diet? |
High forage diet |
|
|
Rate limiting step in biohydrogenation? |
Conversion of trans FA (like vaccenic) to a saturated FA (like stearic acid, 18:0) |
|
|
Why do we need biohydrogenation?? (if we don't have biohydrogenation, something interferes with a critical digestive process) |
PUFA in diet interferes with fibre digestion |
|
|
Is duodenal flow of FAs less than or greater than FA intake? Why? |
Greater than (Duodenal flow of FA > FA intake)
25-50% of FA is associated with microbes |
|
|
Steps of intestinal lipid digestion and absorption? |
1. Lipolysis 2. Emulsification and Micelle formation 3. Absorption (FA transporters, esterification and chylomicron formation) 4. Delivery |
|
|
SCFA/MCFA get delivered to systemic circulation through?? What about LCFA and monoglycerides? |
SCFA and MCFA go directly into portal vein
LCFA and MG get turned into triglycerides and then packaged into chylomicrons to be secreted into the lymph system, which then goes through the thoracic duct and into systemic circulation |
|
|
Chylomicron structure |
Core of non-polar lipids Surface coat of proteins and polar lipids |
|
|
Lipid digestion and absorption abnormality and its causes |
Steathorrhea (excess fat in feces) -Bile salt deficiency -Pancreatic enzyme deficiency -Defective chylomicron synthesis -Lymphatic obstruction |
|
|
Lypolysis definition |
Breakdown of triglycerides by pancreatic lipases to yield monoglycerides and fatty acids |
|
|
Emulsification definition |
Process of decreasing lipid droplet size to form stable suspensions in H20 based solutions |
|
|
Micelle definition |
H20 soluble aggregates of bile acids and lipids |
|
|
Lymphatics from the gut drain into general circulation via the thoracic duct, thereby.... |
Bypassing the liver |
|
|
How are lipids transported? (go through all the steps, byproducts/remnants and receptors) |
From the intestine FA are either transported directly into circulation or indirectly into circulation through lymphatics. Lipoproteins (ex. HDL) and Chylomicrons help transport FA through blood. ApoC receptor helps transport chylomicron and HDL to peripheral tissues where lipoprotein lipase helps in locking onto vascular endothelium and releasing FFA into tissues. After chylomicron releases FAs, turns into a chylomicron remnant which is taken up by the liver through Apo E receptor and endocytosed by hepatocytes |
|
|
What is the name of the receptor for HDL located in the liver? and what does it bind? |
SRB1
A ligand on HDL |
|
|
What happens to excess cholesterol from the tissues? (include ligands and receptors) |
Gets transformed into HDL and then VLDL which has three ligands: B100, C and E. -B100 can bind to ApoB receptor in peripheral tissues -E can bind to ApoE receptor in liver -C can bind to LPL to form FFA in tiissues |
|
|
Function of lipoproteins |
Transport lipids in the blood through stabilizing TG and CE by coating with hydrophillic lipoproteins |
|
|
What does Lipoprotein Lipase (LPL) do? (include location) |
Enzyme bound to endothelial cells of capillaries in peripheral tissue It hydrolyzes TG from chylomicron causing FFA to be released into tissues |
|
|
VLDL where is it from? what does it do? |
(Very Low Density Lipoprotein)
Mainly from liver Assembly, secretion, & metabolism similar to chylomicrons |
|
|
LPL acts on _____ of VLDL to produce ____ or ____ |
TAG IDL LDL |
|
|
What happens to IDL and LDL? |
Endocytosed by liver and other peripheral tissues |
|
|
What does HDL from the liver or intestine do? |
Transports cholesterol from other tissues back to liver |
|
|
Location of TG synthesis |
Primarily in liver and adipocytes Also in cytoplasm and smooth ER |
|
|
Substrates for TG synthesis |
Fatty acids from de novo synthesis, diet or mobilized from fat cells Glycerol-3-P from glycolysis or glycerol released elsewhere |
|
|
Synthesis of TG |
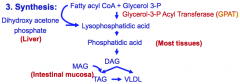
|
|
|
What is TG synthesis also called? |
Lipogenesis or Esterification |
|
|
What is TG breakdown called? |
Lipolysis |
|
|
Location of lipolysis |
Primarily in liver and adipocytes Also in cytoplasm |
|
|
Substrates and enzymes for lipolysis |
Substrate: Lipid droplet Enzymes: Desnutrin, Hormone Sensitive Lipase (HSL) and Monoglyceride Lipase (MGL) |
|
|
Stimuli that promote lipolysis and what they activate |
Epinephrine and Glucagon (activate HSL) Glucocorticoids (activate desnutrin) |
|
|
Stimuli that inhibit lipolysis and what they inhibit |
Insuline (inhibits desnutrin and HSL) |
|
|
Primary sites of De novo synthesis of FAs in cytoplasm: 1. Ruminants 2. Humans and birds 3. Rodents and rabbits 4. Pigs |
1. Adipose and mammary gland 2. Liver 3. Liver and adipose 4. Adipose |
|
|
Sources of NADPH in fat |
1. HMP shunt 2. Isocitrate dehydrogenase 3. Malate dehydrogenase |
|
|
What does ATP from FA do in mitochondria? |
1. Transport FA into mitochondria 2. Beta oxidation to acetyl CoA 3. TCA cycles and ETS: 129 ATP/palmitate molecule |
|
|
Functions of cholesterol |
Most abundant sterol in animals Precursor for steroid hormones and bile acids Component of membranes (and nerves) |
|
|
Sources of cholesterol and which animals use that source |
Diet (carnivores and omnivores) De-novo synthesis (herbivores and carnivores) |
|
|
Steps of cholesterol from diet to circulation |
Cholesterol + bile acids --> intestinal epithelial cells --> free & esterified cholesterol --> Chylomicrons --> lacteals --> thoracic duct --> general circulation |
|
|
De-novo synthesis from? and rate limiting enzyme? |
From acetyl CoA
HMG CoA reductase |
|
|
Sites of cholesterol synthesis |
Primary: liver (50%) and intestine (15%) Secondary: skin, adrenal cortex, ovaries, testes and placenta |
|
|
Cholesterol metabolism: -5 inputs -3 outputs |
Inputs: Receptor mediated, non-receptor mediated, direct transfer, biosynthesis, hydrolysis of stored cholesterol esters
Outputs: Re-esterification, bio-synthesis of bile salts/steroids, transfer to HDL |
|
|
Re-esterification of cholesterol occurs by which enzyme? |
ACAT |
|
|
Transfer of cholesterol to HDL occurs by which enzyme? |
LCAT |
|
|
Lipogenesis 3 pathways and their sources |
1. Glycerol to Phosphatidate (most tissues) 2. DHAP (liver) 3. MAG (intestinal mucosa) |
|
|
Lipogenesis in ruminants: summary 1. Glucose is.... 2. Main sources 3. Sites |
1. Glucose is NOT a precursor for Acetyl CoA 2. Acetate and butyrate in adipose; Glucose in intramuscular adipocytes 3. Primarily adipose in non-lactating animals; adipose and mammary gland in lactating animals |
|
|
Lipogenesis in non-ruminants: summary 1. Glucose is... 2. Main sources 3. Sites |
1. Glucose IS a precursor for FA synthesis 2. Acetyl CoA from mitochondria, transported via citrate 3. Primarily liver in humans, rodents and birds; Adipose in dogs, cats and pigs. Secondarily adipose in humans and rodents; liver in dogs and cats |
|
|
Ketogenesis; what is it |
Ketone body production in mitochondrial matrix of hepatocytes |
|
|
Ketones are energy sources for... |
Heart and muscle Brain during fasting |
|
|
Steps leading to ketogenesis |
OAA is limiting (increase in gluconeogenesis) + increase in Beta-oxidation = Increase in Acetyl CoA that is not used in TCA cycle but is converted to ketone bodies |
|
|
Lipolysis: Beta-oxidation in mitochondria summary 1. provides energy for.. 2. needs what to transfer fatty acyl coA esters 3. (answer to 2) is inhibited by? stimulated by? |
1. Liver, heart and muscle (not brain and RBCs!) 2. Carnitine Shuttle System (CPT-1, CPT-2 & CAT) 3. CPT-1 inhibited by Malonyl Co-A & Insulin; Activated by Glucagon |

