![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
62 Cards in this Set
- Front
- Back
|
Body maintains acid balance through |
Various buffering systems |
|
|
pH and CO2 measurements assess patients |
Acid base status |
|
|
Acid |
Proton donor Give up H+ when dissolved in water |
|
|
Base |
Give up OH- Proton acceptor |
|
|
Pk |
Dissociation constant Describes relative strength of an acid or base Willingness to give up or accept H+ |
|
|
Buffer |
Resists change in pH with addition of small amounts of acid or base or dilution |
|
|
Bicarbonate- carbonic acid system |
Buffer system used by body Pk of 6.1 |
|
|
Body produces much greater quantities of what thru metabolism |
H+ Body is acid producing machine |
|
|
Body's defense against pH change 3 |
Buffering systems Lungs Kidneys |
|
|
Respiratory mechanism (lung) |
CO2 excretion |
|
|
Renal mechanism |
H+ excretion Retain HCO3 |
|
|
Significant drops in pH affect |
Enzyme systems |
|
|
3 buffering systems of the blood |
Bicarbonate carbonic acid Phosphate Plasma proteins |
|
|
pCO2 results from |
More CO2 being found in tissues rather than in nearby blood cells |
|
|
Weak acid of bicarb carbonic acid system |
Carbonic acid H2CO3 |
|
|
Conjugate base of bicarb carbonic acid system |
Bicarbonate HCO3- |
|
|
To maintain normal pH the ratio of bicarb to carbonic acid must be |
20 to 1 |
|
|
Phosphate buffer system |
Plays role in RBC and is involved in exchange of sodium ion in the urine H+ filtrate |
|
|
Plasma protein buffer system |
Most circulating proteins have a neg charge and are capable of binding H+ |
|
|
Regulation of CO2 by lungs- retain |
Retain CO2- accumulates in blood causing and increased H+ cx Caused by decreased ventilation or disease |
|
|
Regulation of CO2 by lungs - eliminate |
Hyperventilation causes CO2 to be released to quickly which decreases the H+ cx |
|
|
1st defense's to changes in acid base status |
Buffer system and lungs |
|
|
Regulation by kidneys |
Excrete variable amounts of acid or base Most effective regulator but takes days to correct Main role is to retain HCO3- |
|
|
CO2 regulation |
Lung regulated Respiratory Acid Gas/volatile |
|
|
HCO3- regulation |
Kidney regulated Metabolic Base Non volatile |
|
|
Henderson hasselbalch equation |
pH= 6.1 + log(HCO3/( 0.03×PCO2)) |
|
|
pH and pCO2 are measured and pk is a constant so |
Bicarbonate HCO3- can be calculated |
|
|
Normal range of pCO2 |
35-45 mmhg |
|
|
Normal range of HCO3- |
22-26 mmol/L |
|
|
Primary disturbance of respiratory acidosis |
Increased pCO2 |
|
|
Primary disturbance of respiratory alkalosis |
Decreased pCO2 |
|
|
Primary disturbance of metabolic acidosis |
Decreased HCO3- |
|
|
Primary disturbance of metabolic alkalosis |
Increased HCO3- |
|
|
Causes of metabolic acidosis |
DiabeticKA, starvation, renal failure, diarrhea, ingestion of acid producing substances (aspirin, ethylene glycol) |
|
|
Compensation for metabolic acidosis |
Increased ventilation to blow of CO2 |
|
|
Causes of respiratory acidosis |
Lung issues, weak rib muscles Barbiturates |
|
|
Compensation mechanism for respiratory acidosis |
Kidneys retain bicarb ions |
|
|
Causes of metabolic alkalosis |
Severe vomiting (lose stomach acid) Too much antacid |
|
|
Compensation for metabolic alkalosis |
Hypoventilation (hold on to CO2) |
|
|
Causes of respiratory alkalosis |
Hyperventilation, fear, anxiety Salicylate poisoning in kids |
|
|
Compensation of respiratory alkalosis |
Kidneys excrete bicarb |
|
|
Measurement of pH |
Potentiametric pH electrodes |
|
|
pCO2 measurement |
Potentiametric ISE |
|
|
pO2 measurement |
Amperometric Measure of current flow |
|
|
HCO3 measurement |
Calculated |
|
|
O2 saturation measurement |
Calculated or co-oximetry |
|
|
Base excess measurement |
Calculated |
|
|
Factors affecting patient pO2 status |
Alveoli destruction (emphysema) Pulmonary edema Airway blockage Inadequate blood supply |
|
|
Base excess |
Amt of H+ ions it takes to return pH to 7.4 >3 is alkalosis <3 is acidosis |
|
|
O2 saturation |
Ratio of O2 bound to hgb to total amt of hgb Assesses ventilation and profusion |
|
|
Oxygen dissociation curve |
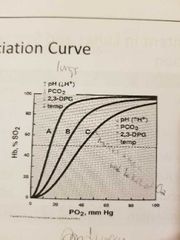
|
|
|
Carboxyhemoglobin |
Hgb that has CO bound to it Hgb has higher affinity for CO |
|
|
Methemoglobin |
Useless for carrying O2 Iron is stuck in 3+ ferric state |
|
|
Venous pO2 and pCO2 |
pO2- 40 pCO2- 46 |
|
|
Arterial pO2 and pCO2 |
pO2- 90 pCO2- 35 to 45 |
|
|
Air bubbles cause |
Increase pO2 and pH Decreased pCO2 |
|
|
Clots cause |
Cannot run |
|
|
Glycolysis causes |
Decreased pH and pO2 pCO2 increased |
|
|
Temperature causes |
1 degree rise causes pH decrease of 0.015 |
|
|
Primary disturbance/ uncompensated |
Abnormal pH plus one other abnormal |
|
|
Fully compensated |
pH is normal The other 2 are out |
|
|
Partially compensated |
All three are out |

