![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
5 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
5 Isomers of hexane |
1. Hexane 2. 2-methylpentane 3. 3-methylpentane 4. 2,2-dimethylbutane 5. 2,3-dimethylbutane |
|
|
|
What is the IUPAC name for: CH3(CH2)7CH3 |
Nonane |
|
|
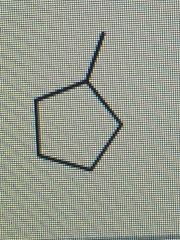
What is the correct IUPAC name for the following cycloalkane: |
Methylcyclopentane |
The ring has five carbon atoms and is therefore a cyclopentane. With only a methyl substituent, no numbering is needed. |
|
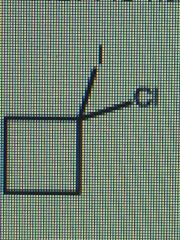
What is the correct IUPAC name for the following cycloalkane: |
1-chloro-1-iodocyclobutane |
The ring has four carbon atoms and is therefore a cyclobutane. There are two halide substituents on carbon 1: a chloro- group and an iodo- group. The location needs to be specified. These are listed in alphabetical order in the name. |
|
|
Why is cyclopropane highly strained? |
1. The cyclopropane ring is unable to pucker and relieve some angle strain
2. The bond angle is 60 degrees, leading to angle strain
3. The cyclopropane CH2 groups are eclipsed with each other, leading to torsional strain |
|

