![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
pH level
|
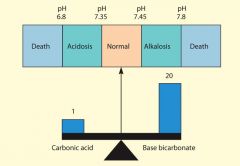
* scale that represents the relative acidity or alkalinity of a substance
* Mathematical expression of H+ concentration (acidity); * pH value higher that 7 is a base or alkaline, it has a lower concentration of hydrogen than hydroxide ions. the more alkaline in a solution the higher the pH value * pH value less than 7 is acidic, a higher hydrogen ion concentration that hydroxide ion concentraions. the higher the hydrogen ion concentraion the lower the pH and the more acid a solution is. * pH of 7 is neutral * All chemical reactions in the body occur at a particular pH level * Deviations in pH impact the outcome of a chemical reaction and thus disturb normal homeostasis * the pH of water is neutral 7.0, it has equal numbers of OH- and H+ ions * the kidneys are the body's most effective regulators of blood pH because they can eliminate much larger amounts of acid than can the lungs, and if necessary can excrete base. * gastric juice (1.6 pH) has a greater concentration of hydrogen ions than saliva (7.7 pH) |
|
|
Cellular metabolism
|
* produces substances that often disturb the normal pH
* When this occurs, the body has mechanisms for regulating the pH of fluids and returning it to normal * The lungs, kidneys, and buffers in the body are the primary regulators to ensure that proper pH and homeostasis are maintained on a daily basis. |
|
|
carbonic anhydrase
|
* an enzyme that accelerates the reaction of carbon dioxide and water combining to form carbonic acid in the kidney tubule
|
|
|
acidosis
|
* condition resulting from an excess proportion of acid in the blood, the blood pH is lower than normal
* anything causing a significant decrease in respirations will produce acidosis |
|
|
buffer
|
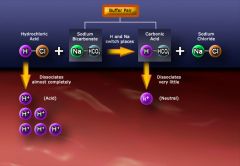
* a chemical substance that prevents a sharp change in the pH of a fluid when an acid or base is added to it
* work primarily by preventing marked changes in pH * Buffers consist of two types of substances and are referred to as "buffer pairs." * one of the main blood buffer pairs is ordinary baking soda and carbonic acid * in order for the buffered pair to function correctly, the concentration of NaHCO3 must be 20 times greater than the concentration of H2CO3 The body has many buffer systems, mainly: * Bicarbonate pair * Plasma and protein pair * Hemoglobin pair * Phosphate buffer pair Buffers are important for adjusting small changes in hydrogen ion concentration. Basically, the buffer pairs work in this manner: * If the H+ concentration in the blood increases, one portion of the buffer pair "removes" H+ from the blood. * If the H+ concentration in the blood decreases, a portion of the buffer pair "donates" H+ to the blood. This give-and-take system, or "switching of positions," allows strong acids and strong bases to become weaker ones and the buffer pairs to maintain a normal pH level in the blood. * if a strong acid such as HCl were added to the buffer pair NaHCO3 and H2CO3, the NaHCO3 would become H2CO3 * if a strong base such as NaOH were added to the buffered pair NaHCO3 and H2CO3, the H2CO3 would become NaHCO3 |
|
|
lactic
|
* acid that builds up in muscles and causes fatigue
* without buffering lactic acid buildup would result in significant H+ accumulation. the resulting decrease in pH and subsequent increase in H+ can produce serious acidosis * lactic acid is one of the most abundant of the fixed acids |
|
|
alkalosis
|
* condition resulting from an excess proportion of alkali in the blood, the blood pH is higher than normal
* anything that causes a significant increase in respirations will eventually cause alkalosis |
|
|
fixed
|
* acid that does not break down to form a gas
|
|
|
base solution
|
* Any solution with a pH above 7 indicates an alkaline
* a compound that produces an excess of OH- ions (or a decrease in H+ ions) * Bases are compounds that, when dissolved in water, reduce the concentration of H+ ions in a solution; sometimes this is accomplished by adding OH- ions * Bases are H+ acceptors. * the higher the hydroxide ion (OH-) concentration, the higher the pH and the more alkaline the solution is |
|
|
acid solution
|
* A solution with a pH below 7 indicates an acid solution
* any substance that, when dissolved in water, contributes to an excess of H+ ions (low pH) (like acids) * Acids donate H+ ions during a chemical reaction. * the higher the hydrogen ion (H+) concentration, the lower the pH and the more acid a solution |
|
|
acid
|
* more acids are added by the body to fluids because of catabolism than bases
* H2CO3 or carbonic acid is the most abundant acid in body fluids. it is made from combining carbon dioxide with water |
|
|
Respiratory Mechanism of pH Control
|
* Respirations help to regulate pH by controlling carbon dioxide (CO@) levels and H2CO3 and hydrogen concentration of blood.
* Carbon dioxide combines with water in the body to form carbonic acid (H2CO3). When respirations are decreased * the body retains carbon dioxide * The carbon dioxide combines with water, forms carbonic acid, and contributes to an excess of H+, which in turn, decreases pH. When respirations are increased: * carbon dioxide is eliminated * there is a decrease in H+ concentration and an increase in pH. * During expiration, CO2 and H2O leave the body in the air. As a result, less CO2 remains in the arterial blood, leaving the lung capillaries, so less of it is available for combining with water to form carbonic acid. Therefore, arterial blood contains less carbonic acid (H2CO3) and fewer hydrogen ions. This means it has a higher pH (7.45) than does venous blood (pH 7.35) |
|
|
Urinary Mechanism of pH Control
|
* The kidneys are the body's best defense against wide variations in blood pH
If the blood is too acid: * the kidney tubules secrete H+ ions from the peritubular capillaries into the filtrate to be removed by the body. * They also conserve sodium bicarbonate by reabsorbing it into the blood, which helps to reduce further the acidity of the blood. If the blood is too alkaline: * the kidney tubules retain more hydrogen ions so that the pH can return to a normal level. |
|
|
carbonic acid
|
* formed when CO2 leaves the blood and enters one of the cells that forms the wall of a distal kidney tubule, it combines with water and forms carbonic acid
* this is a rapid process because the cell contains carbonic anhydrase * when carbonic acid (H2CO3) is formed, some of it dissociates to yield hydrogen and bicarbonate ions. |
|
|
sodium bicarbonate (NaHCO3)
|
* when the hydrogen ions diffuse out of the tubule into the urine. Sodium is displaced by the hydrogen ions and it combines with a bicarbonate ion to form sodium bicarbonate.
* in an effort to assist the process of lowering the acidity of the blood, sodium bicarbonate is reabsorbed by the blood. |
|
|
pH Imbalances
|
Acidosis and alkalosis are the two kinds of pH or acid-base imbalance:
* Acidosis occurs when the blood pH decreases due to an increase in (hydrogen) H+ ion concentration or a decrease in alkalinity or bases. * Alkalosis occurs from a decrease in acidity or an accumulation of bases or alkalinity. * Disturbances to the pH balance are dependent on the proper ratio of sodium bicarbonate (NaHCO3) and carbonic acid (H2CO3) * For pH to remain constant, the ratio of NaHCO3 to H2CO3 must be maintained at 20:1 * the kidneys and the lungs help to maintain pH and this ratio * Disturbances to metabolism can impact the bicarbonate (NaHCO3) element and respiratory disturbances can affect the carbonic acid (H2CO3) proportion in the blood. |
|
|
Metabolic pH Imbalances
|
* Metabolic disturbances affect the bicarbonate element (naHCO3)of the buffer pair
* normal ration of base bicarbonate to carbonic acid is 20:1 * metabolic alkalosis can be caused by severe vomiting * Bicarbonate deficits result in metabolic acidosis * Bicarbonate excesses result in metabolic alkalosis |
|
|
Bicarbonate deficits
|
Bicarbonate deficits result in (compensated) metabolic acidosis.
* Metabolic acidosis is a decrease in pH due to nonrespiratory conditions such as kidney disease, uncontrolled diabetes mellitus, or prolonged vomiting of intestinal contents or diarrhea. * Metabolic acids accumulate when the kidneys fail to excrete sufficient H+ ions, which produces excess keytones in the urine, and causes acidosis * The respiratory system compensates for this by hyperventilation, which removes the CO2 from the body. |
|
|
Bicarbonate excesses
|
Bicarbonate excesses results in (uncompensated) metabolic alkalosis.
* Metabolic alkalosis is an increase in pH caused by nonrespiratory disorders such as an overuse of antacids or bicarbonate-containing drugs, excessive vomiting of stomach contents, or frequent nasogastric suctioning. * The most common cause of metabolic alkalosis is repeated vomiting of stomach contents, which causes a loss of hydrochloric acid. * The respiratory system compensates for this by hypoventilation, which retains CO2 and lowers the pH. |
|
|
Respiratory pH Imbalances
|
* It is important to remember that the respiratory system cannot correct pH imbalances due to a dysfunctional respiratory system
* respiratory disturbances usually have an effect on the H2CO3 part of the buffered pair * It must rely on buffers, the kidneys, or medical management. * anything that causes an excessive increase in the respiration rate causes the pH of the blood to increase * anything that causes an appreciable decrease in the respiration rate causes the pH of the blood to decrease * Carbonic acid excess results in respiratory acidosis * A deficit of carbonic acid results in respiratory alkalosis |
|
|
Carbonic acid excess
|
Carbonic acid excess results in respiratory acidosis
* Respiratory acidosis is caused by any condition that decreases the effectiveness of the respiratory system or causes prolonged hypoventilation such as chronic lung disease or high doses of narcotics. * Anything that interferes with the normal movement of CO2 from the blood to the alveoli, such as occurs in emphysema, can result in respiratory acidosis. * The kidneys attempt to compensate by excreting excess H+ ions in the urine and reabsorbing bicarbonate ions in the blood. * respirations are suppressed so carbon dioxide is retained in the body. |
|
|
carbonic acid deficiency
|
A deficit of carbonic acid results in respiratory alkalosis.
* Respiratory alkalosis is caused by hyperventilation and the resulting decrease in plasma carbon dioxide. * Hyperventilation, caused by fever or severe anxiety causes a loss in CO2, can cause respiratory alkalosis. * The kidneys attempt to compensate for this by decreasing the excretion of H+ ions and increasing the excretion of bicarbonate ions in the urine. |
|
|
Disorders of Metabolism and Respirations
|
* The respiratory system and the kidneys can both cause and correct pH imbalances.
* The delicate balance that must be maintained to keep the body at a proper pH level gives us a real appreciation for the chemistry that must be maintained for survival. The table below summarizes disorders of metabolism and respirations. |
|
|
Disorders of Metabolism and Respirations (table)
|
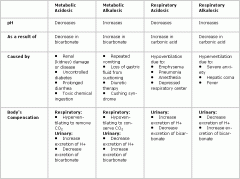
.
|
|
|
acid-base balance
|
* keeping the concentration of hydrogen ions (H+) in body fluids relatively constant
* one of the most important of the body's homeostatic mechanisms |
|
|
pH
|
* the term pH followed by a number indicates a solution's relative hydrogen ion concentration compared to hydroxide concentration
* pH is an acronym for "power of H+" * water and all water solutions contain hydrogen ions (H+) and hydroxide ions (OH-) * the kidney is more effective in pH regulation that the lung because it can remove base which the lung cannot |
|
|
chemical pH control mechanism
|
* based on buffers
* acts immediately to help prevent harmful swings in pH when added acids or bases enter body fluids. |
|
|
physiological pH control mechanism
|
* provided for by the lungs and kidneys when the chemical pH control mechanism is unable to stabilize the pH
|
|
|
bicarbonate loading
|
* ingesting large amounts of sodium bicarbonate (NaHCO3) to counteract the effects of lactic acid buildup
* used by athletes to prevent muscle soreness and fatigue that accompany strenuous exercise. * can result in diarrhea |
|
|
ketone bodies
|
* acidic substances in the blood results from the excessive metabolism of fats, most often in those people with uncontrolled type I diabetes.
* associated with cellular metabolism of fats |
|
|
diabetic ketoacidosis
|
* the accumulation of ketone bodies results in a condition called diabetic ketoacidosis
* causes the blood to become dangerously acidic |
|
|
metabolic alkalosis
|
* the most frequent and serious condition that can occur from repetitive vomiting over time
* therapy to restore the buffer pair ratio to normal includes IV administration of chloride-containing solutions such as normal saline. |
|
|
fixed acid
|
* acids that do not break down to form a gas
* sodium bicarbonate (naHCO3) or ordinary baking soda is one of the main buffers of the most abundant "fixed acids" in blood * when a fixed acid is buffered in the blood, the amount of NaHCO3 in the blood decreases * when a fixed acid is buffered in the blood, the amount of hydrogen ions in the blood increases * when a fixed acid is buffered in the blood, the amount of H2CO3 in the blood increases * when a fixed acid is buffered in the blood, the pH of the blood decreases |
|
|
distal tubule
|
* the part of the nephron that is important in regulation of blood pH
|
|
|
NaH2PO4
|
* the end product that leaves the body in the urine when Na2HPO4 is used by the kidney to remove hydrogen ions from the blood
|
|
|
NH4Cl
|
* the end product that leaves the body in urine when ammonia is used by the kidney to remove hydrogen ions from the blood
|

