![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
39 Cards in this Set
- Front
- Back
|
Nitrogen with 4 groups salt
|
Quaternary ammonium salt
N+R4 X- |
|
|
Common name for phenylamine
|
Aniline
Ph-NH2 |
|
|
Properties of chiral amine (hybridization, etc.)
|
an amine with 3 diferent substituents is sp3 hybridized, tetrahedral and chiral.
HOWEVER, the amine will invert via SN2 reaction where it is temporarily sp2 hybridized, then returns to sp3 in reversed RS. Barrier to this is similar to rotation about C=C bond |
|
|
Basicity of amine (how is it measured)
|
RNH2 + H2O(acts as an acid) <> RNH3+ + OH-
a salt The bacisity of RNH2 is measured via the ka of RNH3+ High Ka, low pKa- weak base Low Ka, high pKa- strong base |
|
|
Basicity trends for:
Ammonia, Primary, Sec, Tert, Aryl Amine Heterocycles |
pKa:
ammonia- 9.2 prim,sec,tert amines range of 10-11 (stronger than ammonia) arylamine, heterocycles 0>6 weak bases pyrolle almost 0 |
|
|
Bacisity of Amides,
why? |
Not basic. Do not protonate
resonance form makes them electron poor, bad nucleophiles |
|
|
Purification of amine + neutral compound(e.g. ketone)
dissolved in organic solvent |
Add HCl, Amine is now mostly in its protonated salt form in the aqueous layer. Separate layers and neutralize > pure amine
|
|
|
Basicity of aryl amines, why less basic
What do substituent groups do? |
Many resonance forms as NH2 is electron donating in the form of resonance. Protonation gets rid of resonance and destabilizes it.
Activating groups are electron donating, will make aryl-amine more basic Deactivating groups lower basicity |
|
|
Amine synthesis from RCOOH
|
RCOOH + SOCl2 >> + NH3> RCONH2 + LiALH4,Ether >
|
|
|
Preparation of Aryl Amines
Starting groups? |
Aromatic group nitrated:
Aryl + HNO3 + H2SO4 |
|
|
Prep of amine from alkyl halide
1st degree 2nd degree 3rd degree type of reaction does it stop? |
NH2 attacks RX > RNH3+ X- > NaOH > RNH2 primary
RNH2 attacks RX > R2NH2+ X- > NaOH> R2NH secondary R2NH attacks RX> R3NH+ X- > NaOH> R3N tertiary R3N attacks RX> R4N+ X- (quarternary ammonium salt) SN2 reaction DOES NOT STOP! mostly will be primary and secondary though |
|
|
? degree Amine prep from azide ion
Structure? requirement for akyl? |
RX + NaN3/Ethanol > RN=(N+)=(N-)
azide ion (N3)- attacks R, SN2 RN=(N+)=(N-) no longer nucleophilic, RN=(N+)=(N-) + LiAlH4,ether/H2O> RNH2 1st degree, R must be 1st or 2nd degree |
|
|
Gabriel synthesis of ? degree amines
Name of starting func. group? alternate removal method? |
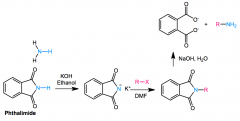
RCONHCOR is an IMIDE
KOH/ethanol removes proton, nucleophile attacks R-X/DMF. completed via NaOH, H2O (hydrolisis) ALTERNATIVELY H2NNH2 and heat for final step, takes form CONHNHOC |
|
|
Reductive Amination
process/mech |
Ketone/Aldehyde + NH3/ H2/Ni(or NaBH4)
RCOR' > RCH(NH2)R' can be repeated to yield secondary or tertiary amines Features: Ammonia attacks carbonyl carbon. OH and H are expelled. NH forms double bond, water leaves NABH4 reduces to NH2 |
|
|
Alternate reagent for Reductive amination (why)
|
NaBH4 can reduce C=O so imine must be formed first
NABH3CN/methanol can only reduce C=N, instant reductive amination in one step! |
|
|
Hoffman Rearrangement
Reactants/products purpose Mechanism name/structure intermediate what catalyzes decarboxylation |
Convert amide into primary amine
RCONH2 + NaOH,BR2/H2O > RNH2 + CO2 Base removes acidic NH proton N- attacks BR2 yielding RCONHBR + Br- OH removes other H, rearranges to form RC(O-)=NBr O- returns charge, Br leaves, R, bonds to N (R-N=C=O + Br -) N attacks H-OH HO- attacks C HOC(=O)-NHR N attacks HOH, H+ leaves, RNH2 + CO2 + OH- intermediate R-N=C=O is an isocyanate decarboxylation via H2O catalyst |
|
|
Reduction of RNO2 to RNH2
|
H2/Pt ethanol if no other groups can be reduced
if carbonyls present, use SNCl2,Acid, then NaOH |
|
|
Curtis rearrangment
purpose starting/ending mechanism? |
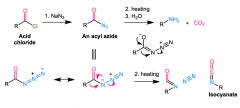
Converts acyl azide (RCON=(N+)=(N-) into RNH2 + CO2 + N2
RCOX + NaN3 > RCON3 azide rearranges to (N-)-(N+)(trip)N Heating: R attacks N, Nattacks C, N2 leaves isocyanate (R-N=C=O) decarboxylated via H2O |
|
|
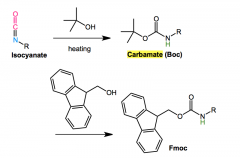
Curtis rearrangement application in proteins?
|
Iocyanate + heat + (tert)-butyl-acohol or other to form Boc and Fmox
|
|
|
Acylation to form amide
what kinds of amines can be used? whats can you do next? |
RCOX + NH3 + pyridine solvent > RCONH2 + HCl
RCOX + RNH2 +solvent> RCONHR + HCl RCOX +R2NH + solcent > RCONR2 + HCl ammonia, primary and secondary react LiALH4 to form amines |
|
|
Hoffman elimination
purpose? Mechanism? |
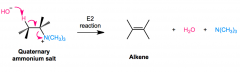
E2 reaction
gets rid of amine, forms alkene R-C(NH2)H-CH3 + Excess (CH3I)>> + Ag2O/H2O,heat > R-CH=CH2 OH- removes Hydrogen, C=C bond forms, quaternary ion leaves H is taken from less substituted, less hindered C (non-zaitsev) |
|
|
Electrophilic substitution of Aryl amines
Br2 + ? ? How is it controlled? |
strongly ortho para directing, strongly activating
PH-NH2 + Br2 + H20 yields (2,4,6)tribromoanniline to avoid this, reactions controlled via conversion to amide |
|
|
NHAc Protecting group
structure how is it formed reactions |
4-methyl-anniline + Ac2O(acetic anhydride/pyridine)>
yields methyl amide H3CCONH-toluene Amide N lone pair is delocalized, so Br2 leads to monobromination at ortho position. Subsequent hydrolysis via NaOH,H2O yields 2-bromo-4methyl-anniline |
|
|
Friedel crafts with Amide protecting group
|
Anniline + Acanhydride/pyridine > benzene amide > Ph-COCl/AlCl3 and subsequent hydrolisis via NaOH,H2O yields 4-aminobenzophenone
|
|
|
Sandmeyer reaction (functional group?)
useful for alkyl, aryl or both? what is this process called? purpose? example different Nus |
diazonium salt Ar-(N+)tripN X-
only aryl diazotization anniline + HNO2 + H2SO4 > Ph-(N+)trip(N) -(HSO4) +2H2O diazonium ion can be replaced by Nu in a sub rxn HX + CuX is used unless X=I p-methylaniline +HNO2/H2SO4 > pmethyldiazonium salt +HBr/CuBr> p-bromotoluene If X=I, no need for CuX, just add NaI to diazonium salt Also KCN can be used with CuCN to yield nitrile, then hydrolisis to add CO2H NOT AN SN reaction |
|
|
diazonium salt mechanism
|
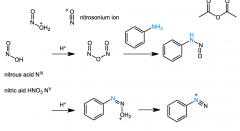
HNO2 forms like acetic anhydride
Ph-NH-N=O + H+> Ph-N=N-O+H2 > Ph-(N+)tripN |
|
|
Phenol formation
|
Ar-NH2 + HNO2 +H2SO4 > diazonium salt
+ Cu2O/(Cu(NO3)2,H2O > Ar-OH |
|
|
Using Diazonium, then getting rid of it
|
Annilin + Br2> tribrominated anniline
+HNO2 + H2SO4 yields diazonium-benzene (2,4,6)tribromo + H3PO2 > 2,4,6tribromobenzene |
|
|
Diazonium Coupling
what position? mechanism? |
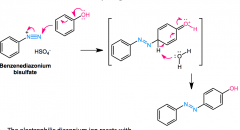
Diazonium salt + Ph-Y (Y=OH- or -NR2) in H2O> Ph-N=N-Ph-Y
will normally go para, but will go ortho if blocked |
|
|
Azo dyes
structure? function |
Diazonium coupling where y= -N(Me)2
to give Ph-N=N-Ph-N(Me)2 extended conjugated network absorbs visible light, works as dye |
|
|
friedel crafts
|
Ph + RCOCl + AlCl3 > Ph-COR
|
|
|
Pyrolle basicity and reactivity
reaction with Br2 |
RNH+ pka of .4
amine and conjugated diene N is sp2 hybridized, so lone pair is kept in p orbital, delocalized. VERY STABLE Aromatic despite 5 memebered When added to Br2, double bond attacks Br2 at C2 position (cation at C3 involves 3 resonance forms). If attack at C3, cation at C2 involves only 2. Br- gets rid of H at C2. substituted Br at C2 |
|
|
Pyridine properties
basicity reactivity |
Sp2 hybridized, lone pair in sp2 orbital perpindicular to pi-bond system. More basic than pyrolle, but sp2 orbital has more s character, electrons less reactive.
substantial dipole moment due to lone paid and inductive effect of N. Very electron poor ring, deactivated |
|
|
Pyrimidine
|
Less basic than pyridine because of inductive effects of second N
|
|
|
Imidazole
|
pKa of 7, more basic than pyrolle
|
|
|
indole
reactivity |
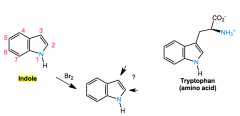
like a pyrrole but with added phenyl group
electrophillic addition takes place at C3 |
|
|
curtius rearrange for Boc
|
|
|
|
Diels Alder
|
conjugated diene + substituted alkene
aldehyde, ester, anhydride, nitrile cyclic transition state forms ring simultaneously sp2 to sp3 hybridization |
|
|
knovenegel
|
malonic ester + aldehyde no alpha h NaOet, dehydration, then h30+ to yield R-C=C-COOH
|

