![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
|
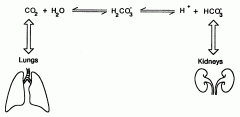
Bicarbonate-carbonic acid buffer system |
|
|
|
CO2 + H2O → H2CO3 → H+ + HCO3-
(Describe what's happening in this reaction) |
In red blood cells, the enzyme carbonic anhydrase catalyzes the conversion of dissolved carbon dioxide to carbonic acid, which rapidly dissociates to bicarbonate and a free proton |
|
|
What does the bicarbonate-carbonic acid buffer system do? |
|
|
|
Bicarb equation |
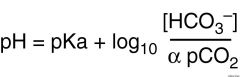
|
|
|
Ratio of bicarbonate to carbonic acid (or partial CO2)? |
20:1 |
|
|
Define acidosis |
blood pH < 7.35 |
|
|
Define alkalosis |
blood pH > 7.45 |
|
|
R.O.M.E. |
Respiratory alkalosis or acidosis = pH and CO2 move in opposite direction
pH ↓ & CO2↑ = Acidosis pH ↑ & CO2↓ = Alkalosis
Metabolic alkalosis or acidosis = pH and bicarb move in equal direction
pH ↓ & HCO3 ↓= Acidosis pH ↑ & HCO3 ↑= Alkalosis |
|
|
Causes of respiratory acidosis |
|
|
|
Causes of respiratory alkadosis |
|
|
|
Causes of metabolic acidosis |
|
|
|
Causes of metabolic alkadosis |
|
|
|
Effect of increased respiration on blood pH? |
Increased respiration = breathing out CO2 = decreases CO2 (acidic) = increases pH |
|
|
Effect of decreased respiration on blood pH? |
Decreased respiration = not breathing out as much CO2 = increased CO2 (acidic) = decreases pH |
|
|
How do kidneys affect blood pH? |
Decrease acid by excreting H+ (gets rid of acid)
Decrease acid by secreting bicarbonate into blood (increases base in blood) |

