![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
94 Cards in this Set
- Front
- Back
|
Carbohydrates (saccharides) are the single most abundant class of biomolecules in nature.Carbohydrates have a variety of functions:
|

|
|
|
Carbohydrates are either polyhydroxyaldehyde or polyhydroxyketone, polyfunctional compounds, what are they ?
|
Carbohydrates are either polyhydroxyaldehyde or polyhydroxyketone, polyfunctional compounds, containing two or more hydroxyl groups and an aldehyde or ketone functional group.
|
|
|
Carbohydrates can be further classified as Monosaccharides. Explain?
|
(glucose, fructose, ribose) sugars, sweet tasting
have important biological roles themselves and serve as building blocks for oligosaccharides and polysaccharides |
|
|
Oligosaccharides: smaller than a polysaccharide but larger than a monosaccharide (10-20 monosaccharide residues) Show me some examples?
|
Disaccharide: (lactose, sucrose) sugars, sweet tasting
Trisaccharide Tetrasaccharide |
|
|
Polysaccharides: hundreds to thousands of monosaccharide units bonded together. What are some examples?
|
starch, cellulose, glycogen, chitin
|
|
|
IUPAC names are not commonly used for carbohydrates.
Most common names of carbohydrates end in what? |
IUPAC names are not commonly used for carbohydrates.
Most common names of carbohydrates end in –ose |
|
|
What are some examples of carbohydrates ending in -ose?
|
Glucose, fructose, lactose, sucrose, cellulose
|
|
|
Most polysaccharides do not end in –ose. Give me some examples?
|
Starch, chitin, glycogen
|
|
|
Dietary carbohydrates are classified as?
|
Simple: monosaccharides and disaccharides
Complex: polysaccharides |
|
|
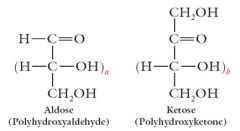
Monosaccharides have one of the following structures. Explain.
|
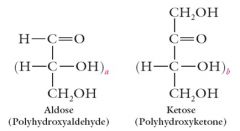
|
|
|
For monosaccharides of biological importance, a = 1-4 and b= 0-3.
In the aldoses, the carbonyl is C1; in the ketoses, the carbonyl is C2 |
|
|
|
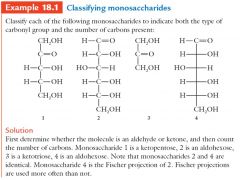
Monosaccharide names combine two kinds of prefixes with –ose. A 5-carbon sugar containing a ketone group is called a ketopentose
|

|
|
All D-aldoses have the hydroxyl farthest from the carbonyl group pointing to the right (blue hydroxyl).
|
|
|
|
All D-ketoses have the hydroxyl group farthest from the carbonyl carbon pointed to the right (blue hydroxyl)
|
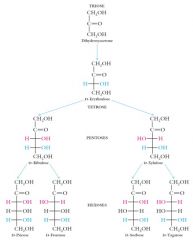
|
|
|
Each D-aldose and D-ketose theoretically has an L-enantiomer, however, these are almost what?
|
Each D-aldose and D-ketose theoretically has an L-enantiomer, however, these are almost never found in nature.
|
|
|
Degradative and synthetic enzymes almost always recognize only the D-enantiomers of sugar molecules a principle of what?
|
Degradative and synthetic enzymes almost always recognize only the D-enantiomers of sugar molecules (principle of chiral recognition).
|
|
|
Some Important Monosaccharides
D-Glucose is the most what? |
D-Glucose is the most abundant monosaccharide in nature.
|
|
|
Some Important Monosaccharides
What is D- Glucose also called is? |
Also called blood sugar or dextrose
|
|
|
Some Important Monosaccharides
Where is D-Glucose found? |
Found in combined forms: starch, cellulose, glycogen, chitin, lactose, and sucrose.
|
|
|
Some Important Monosaccharides
D-Fructose (levulose) is bonded to glucose to form sucrose, the sugar in what? |
D-Fructose (levulose) is bonded to glucose to form sucrose, the sugar in fruits and table sugar.
|
|
|
Some Important Monosaccharides
D-Galactose is bonded to glucose to form lactose, the sugar in what? |
D-Galactose is bonded to glucose to form lactose, the sugar in milk.
|
|
|
D-ribose, D-xylose, and 2-Deoxyribose are important pentoses.
Components of what? |
D-ribose, D-xylose, and 2-Deoxyribose are important pentoses.
D-ribose: Component of RNA D-xylose: Component of some plant polysaccharides 2-Deoxyribose: Component of DNA |
|
|
In 2-deoxyribose, the –OH at C2 is replaced by –H. It is an example of what?
|
In 2-deoxyribose, the –OH at C2 is replaced by –H. It is an example of a deoxysaccharide or deoxysugar.
|
|
|
Cyclic Hemiacetal Structures
The –OH group of an alcohol and the carbonyl group of an aldehyde or ketone can undergo an acid catalyzed reaction to form a hemiacetal: |
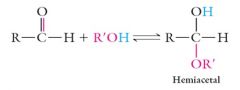
|
|
|
Sugar molecules contain both an alcoholic functional group and an aldehyde or ketone functional group and form cyclic hemiacetals.
In the case of D-glucose, the ring formed is called a what? |
Sugar molecules contain both an alcoholic functional group and an aldehyde or ketone functional group and form cyclic hemiacetals.
In the case of D-glucose, the ring formed is called a pyranose ring |
|

|
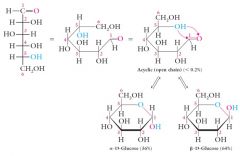
|
|
|
The formation of the hemiacetal structure converts the carbonyl carbon into a new tetrahedral stereocenter.
|
|
|
|
|
This carbon is called the hemiacetal carbon. It can be recognized because it has two different oxygen groups attached:
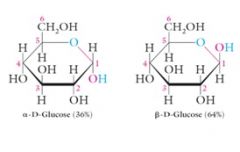
-OR an ether linkage -OH an alcohol Two stereoisomers are possible at the hemiacetal carbon called Alpha and Beta configurations. D-glucose consists of an equilibrium mixture of these two hemiacetals: 36% Alpha and 64% Beta |
|
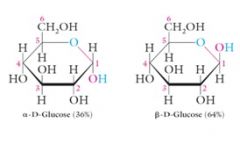
|
This carbon is called the hemiacetal carbon. It can be recognized because it has two different oxygen groups attached:
-OR an ether linkage -OH an alcohol Two stereoisomers are possible at the hemiacetal carbon called Alpha and Beta configurations. D-glucose consists of an equilibrium mixture of these two hemiacetals: 36% Alpha and 64% Beta |
|
|
These stereoisomers are diastereomers of each other and are called what?
|
These stereoisomers are diastereomers of each other and are called anomers.
|
|
|
The alpha anomer of D-glucose has a cis relationship between the –OH at C1 and the –CH2OH at C5.
The Beta anomer of D-glucose has a trans relationship between the –OH at C1 and the –CH2OH at C5. |
|
|
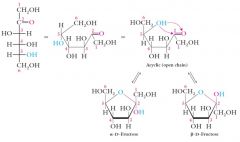
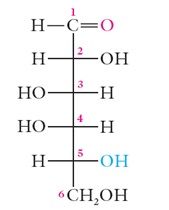
The acyclic form of glucose (open-chain structure) contributes less than 0.2% of the equilibrium mixture and is not stable compared to the hemiacetal forms of what?
|
The acyclic form of glucose (open-chain structure) contributes less than 0.2% of the equilibrium mixture and is not stable compared to the hemiacetal forms of glucose (alpha 36%; beta 64%).
|
|
|
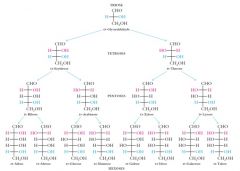
Repeat the concept checklist for Hexoses and pentoses.
|

|
|
|
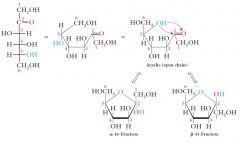
D-Fructose and other ketohexoses form 5-membered cyclic hemiacetals called furanoses.
|
|
|
|
Note the trans relationship in the alpha anomer and the cis relationship in the beta anomer between the –OH and –CH2OH groups.
|
|
|
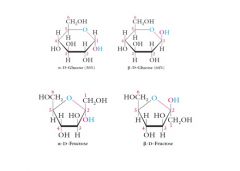
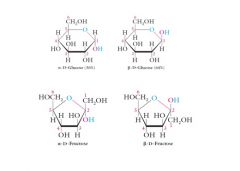
These 5-membered and 6-membered cyclic hemiacetal structures are called Haworth structures or Haworth projections. Explain?
|
|
|
|
The bold bonds at the bottom of each structure are meant to appear closer to the viewer (above the plane of the screen) than the C-O or O at the top of the ring which is meant to appear farther from the viewer (below the plane of the screen).
Haworth projections are not always drawn with bold bonds. |
|

|
|
|
|
Show me the 3 step process for D-glucose?
|
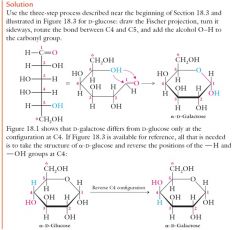
|
|
|
What are some Chemical and Physical Properties of Monocaccharides?
|
Monosaccharides are crystalline solids at room temperature and are very soluble in water where they can form highly viscous solutions.
Monosaccharides are slightly soluble in alcohols (methanol, ethanol) and are insoluble in less polar solvents (ethers, hydrocarbons). Almost all monosaccharides taste sweet |
|
|
What are some other Chemical and Physical Properties of Monocaccharides?
|
A solution of a monosaccharide consists of the anomer, anomer, and acyclic structure. These structures interconvert rapidly and form an equilibrium mixture.
Usually, only a single anomer is drawn which represents both, when drawing the structure of a mono- or disaccharide in a chemical equation. |
|
|
Show me the sweetness table in relation to Sucrose?
|
|
|
|
Show me the sweetness table in relation to Sucrose?
|
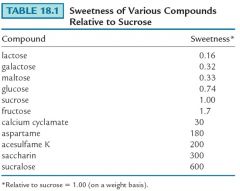
|
|
|
The Oxidation of the Aldehyde Group
The aldehyde group of an aldose can be oxidized by Cu2+ complexed with citrate ion in alkaline solution (Benedict’s solution) to a carboxylic acid. This oxidation occurs with the acyclic (open chain) form of the aldose and not with the hemiacetal form. The –COOH will actually be ionized, -COO-, in alkaline solution |
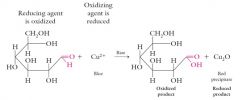
|
|
|
Aldoses and ketoses are called reducing sugars because they are able to reduce Cu2+ in what?
. |
Aldoses and ketoses are called reducing sugars because they are able to reduce Cu2+ in Benedict’s reagent.
|
|
|
The glucose oxidase test is specific for what, not all aldoses and ketoses?
|
The glucose oxidase test is specific for D-glucose, not all aldoses and ketoses.
|
|
|
D-glucose is oxidized by O2 to D-gluconic acid and hydrogen peroxide by the enzyme ?
|
D-glucose is oxidized by O2 to D-gluconic acid and hydrogen peroxide by the enzyme glucose oxidase
|
|
|
The H2O2 produced, oxidizes added o-toluidine to give what products?
|
The H2O2 produced, oxidizes added o-toluidine to give colored products.
|
|
|
Acetal Formation: The Production of Glycosides
Monosaccharides can be converted into glycosides by the acid-catalyzed reaction of the hemiacetal –OH group with an alcohol. Reaction occurs preferentially at the hemiacetal –OH because it is the most reactive hydroxyl present. |
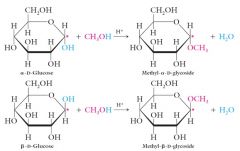
|
|
|
Acetals of carbohydrates are called what, and the C-O-C bond at the anomeric carbon is called a what linkage?
|
Acetals of carbohydrates are called glycosides, and the C-O-C bond at the anomeric carbon is called a glycosidic linkage
|
|
|
In the acid-catalyzed laboratory reaction, a mixture of both alpha and beta glycosidic linkages is formed. In living systems one of the other glycosidic linkage is enzymatically formed, never what?
|
In the acid-catalyzed laboratory reaction, a mixture of both and glycosidic linkages is formed. In living systems one of the other glycosidic linkage is enzymatically formed, never both.
|
|
|
Glycosides are not reducing sugars because what? For the same reason, they do not undergo mutarotation.
|
Glycosides are not reducing sugars because they are not in equilibrium with an open chain form. For the same reason, they do not undergo mutarotation.
|
|
|
In the presence of water and an acid catalyst or the appropriate enzyme, glycosides can be hydrolyzed to what?
|
In the presence of water and an acid catalyst or the appropriate enzyme, glycosides can be hydrolyzed to the saccharide and alcohol.
|
|
|
Polysaccharides consist of monosaccharides linked together by glycosidic bonds. The presence of alpha versus beta glycosidic linkages in polysaccharides is important to their what?
|
Polysaccharides consist of monosaccharides linked together by glycosidic bonds. The presence of alpha versus beta glycosidic linkages in polysaccharides is important to their structure and function.
|
|
|
Other Derivatives of Monosaccharides
Phosphate Esters: Ribose-5-phosphate is the building block in the synthesis of RNA. |
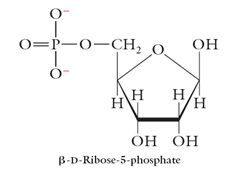
|
|
|
2-Deoxyribose-5-phosphate is the building block in the synthesis of what?
|
2-Deoxyribose-5-phosphate is the building block in the synthesis of DNA.
|
|
|
Glucose-6-phosphate and Fructose-6-phosphate are intermediates in the metabolism of what?
|
Glucose-6-phosphate and Fructose-6-phosphate are intermediates in the metabolism of saccharides.
|
|
|
Acidic Sugars:
D-Glucuronic acid: The –CH2OH group has been oxidized to –COOH. A building block for hyaluronic acid. |
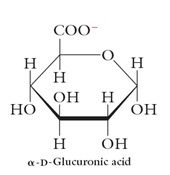
|
|
|
Amino Sugars:
D-Glucosamine: The –OH group at C2 has been replaced by –NN2. |
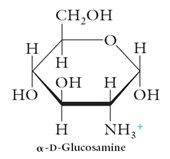
|
|
|
N-acetyl-D-glucosamine: The building block for chitin, the exoskeleton of crustaceans. A building block for hyaluronic acid.
|
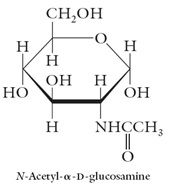
|
|
|
What are some properties of Disaccharides?
|
Disaccharides are two monosaccharides joined by a glycosidic linkage.
The glycosidic linkage in a disaccharide results from a dehydration reaction between the anomeric hydroxyl group of one monosaccharide and one of the alcoholic groups of the other monosaccharide. To characterize a disaccharide |
|
|
Characterize a disaccharide?
|
|
|
|
Maltose
Also known as corn sugar, or malt sugar. Produced by the partial hydrolysis of starch by the enzyme amylase. In maltose, two D-glucose molecules are linked by an alpha(1-4) linkage. |
|
|
|
Maltose
Also known as corn sugar, or malt sugar. Produced by the partial hydrolysis of starch by the enzyme amylase. In maltose, two D-glucose molecules are linked by an alpha(1-4) linkage. |

|
|
|
Because maltose has a free hemiacetal –OH, it is a reducing sugar.
The in -D-maltose refers to the -anomer of maltose, not the configuration of the anomeric hydroxyl in the glycosidic bond. |
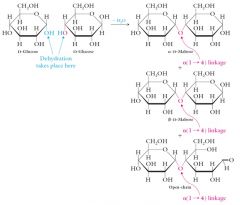
|
|
|
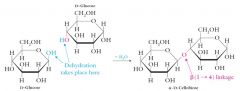
Cellobiose is a product of the partial hydrolysis of cellulose.
Cellobiose is identical to maltose except that the two glucose molecules are connected by a beta-anomeric linkage rather than an alpha-anomeric linkage. Cellobiose is a reducing sugar. |
|
|
|
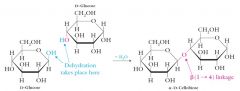
Lactose, or milk sugar, constitutes about 4-8% of mammalian milk.
Lactose contains galactose and glucose joined by a beta(1-4) glycosidic linkage. Lactose is a reducing sugar. Lactose intolerance and galactosemia are two significant hereditary diseases involving lactose consumption. |
|
|
|
The most abundant disaccharide in nature.
Sucrose is formed by acetal formation between the hemiacetal –OH groups of both alpha-D-glucose and beta-D-fructose. The linkage is alpha,beta(1-2): Because both hemiacetal –OH groups are involved in the glycosidic linkage, sucrose is not a reducing sugar. |
|
|
|
The most abundant disaccharide in nature.
Sucrose is formed by acetal formation between the hemiacetal –OH groups of both alpha-D-glucose and beta-D-fructose. The linkage is alpha,beta(1-2): Because both hemiacetal –OH groups are involved in the glycosidic linkage, sucrose is not a reducing sugar. |
|
|
|
What are some of the properties of The Digestion and Absorption of Carbohydrates?
|
The sugars maltose (from starch digestion), lactose, and sucrose cannot be directly absorbed from the intestinal track. Each must first be enzymatically hydrolyzed to the constituent monosaccharides:
Maltose: maltase Lactose: lactase Sucrose: sucrase Enzymes are usually named by adding the suffix –ase to the name of the compound undergoing the reaction. |
|
|
Polysaccharides contain large numbers (hundreds or thousands) of monosaccharides residues (repeat units) bonded together.
Starch and glycogen serve as storage forms for D-glucose. Cellulose and chitin serve as biological structural materials. Polysaccharides differ in the following ways: |

|
|
|
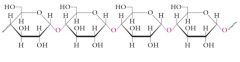
Starch and glycogen are storage polysaccharides for D-glucose.
Starch: Plants Amylose: 10-30%, unbranched, alpha(1-4) linkages |
|
|
|
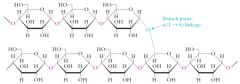
Amylopectin: 70-90%, branched, alpha(1-4) linkages and alpha(1-6) branches, branches have branches, MW 1,000,000 or more.
|
|
|
|
What are some properties of glycogen?
|
Glycogen: Animals
Glycogen is similar to amylopectin but more highly branched. Amylose, amylopectin, and glycogen all contain a single free hemiacetal hydroxyl and hundreds or thousands of hemiacetal glycosidic linkages. The percentage of free hemiacetal –OH groups is so small that none of these molecules give a positive test with Benedict’s solution. They are all non-reducing sugars. |
|
|
Explain the concept of polysaccharides?
|

|
|
|
Explain the concepts of starch and glycogen in digestion and metabolism
|
Starch is the principle carbohydrate in our diet and is digested to D-glucose:
Amylase hydrolyzes amylose and parts of amylopectin to maltose in the digestive tract. Maltase cleaves maltose to D-glucose. The result of amylase digestion of amylopectin is dextrin which contains the remaining alpha(1-6) linkages. Dextrin is hydrolyzed to dextrinase to D-glucose. |
|
|
What are some other concepts of glycogen in digestion?
|
Some of the D-glucose from starch is used immediately for energy (glycolysis).
The excess D-glucose is stored in the liver and skeletal muscles as glycogen (glycogenesis). Any D-glucose still in excess is converted to fat and deposited in the fat tissues. |
|
|
When required for energy or biosynthesis, D-glucose is released from a glycogen molecule (glycogenolysis).
Removal of D-glucose from glycogen can be very rapid because it can be removed from all of the tips of the glycogen branches simultaneously: |

|
|
|
Cellulose is a structural polysaccharide, and is the most abundant organic compound in the biosphere (50% of all organic carbon).
Cellulose is a linear polymer of D-glucose monomers connected with beta(1-4) linkages. |
|
|
|
Cellulose is a structural polysaccharide, and is the most abundant organic compound in the biosphere (50% of all organic carbon).
Cellulose is a linear polymer of D-glucose monomers connected with beta(1-4) linkages. |
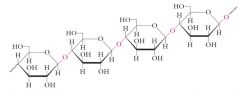
|
|
|
What are some properties of cellulose?
|
The properties of cellulose are much different from those of -amylose or starch.
Starch swells and forms a colloidal suspension when placed in water. Cellulose (wood) is insoluble and retains its shape and most of its physical strength when placed in water. Cellulose molecules exist in an extended chain conformation and pack side to side to form ribbons. The ribbons pack side to side and on top of each other to form fibers. All of the cellulose molecules are held together in a fiber by intermolecular hydrogen bonding. |
|
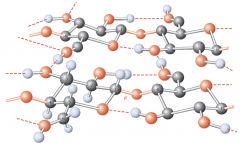
|

|
|
|
What are some characteristics of cellulose?
|
Humans cannot digest cellulose because we lack the enzyme cellulase which cleaves beta(1-4) linkages. Even so, cellulose in the diet has some beneficial effects.
The main nutritional carbohydrate for grazing animals is cellulose (grass and other plants). These animals cannot digest cellulose directly, but have symbiotic microorganisms in their digestive tracts that secrete cellulase into the animal’s digestive tracts. Starch can be differentiated from cellulose by adding a few drops of I2 solution. I2 forms a dark blue solution in the presence of starch which does not form with cellulose. |
|
|
What are some properties of Cell Recognition: Glycolipids and Glycoproteins?
|
Cells interact with and recognize other cells through a process called cell recognition.
Cell recognition is accomplished through saccharides attached to the cell surfaces. These saccharides, usually oligosaccharides, are present as glycolipids and glycoproteins. The lipid or protein part of the molecule is integrated into the cell-membrane structure with the saccharide part located on the external membrane surface. |
|
|
Plant and animal life is possible only because of photosynthesis.
Photosynthesis traps the energy of sunlight and uses it to transform carbon dioxide and water into organic compounds and molecular oxygen. About 20% of photosynthesis on Earth takes place in land plants and 80% takes place in the oceans. |
|
|
|
Plant and animal life is possible only because of photosynthesis.
Photosynthesis traps the energy of sunlight and uses it to transform carbon dioxide and water into organic compounds and molecular oxygen. About 20% of photosynthesis on Earth takes place in land plants and 80% takes place in the oceans. |
|
|
|
Plant and animal life is possible only because of photosynthesis.
Photosynthesis traps the energy of sunlight and uses it to transform carbon dioxide and water into organic compounds and molecular oxygen. About 20% of photosynthesis on Earth takes place in land plants and 80% takes place in the oceans. |

|
|
|
The energy stored in the carbohydrates created through photosynthesis ultimately supplies the energy for all animal life on earth.
While utilizing carbohydrates for energy, animals recombine the carbohydrates with oxygen and return carbon dioxide and water to the environment, thus completing a cycle. Plants and animals are thus interdependent through a carbon cycle and an oxygen cycle: |
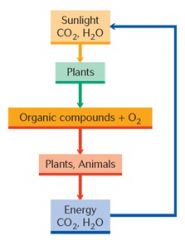
|
|
|
Explain biochemisty.
|
Biochemistry is the study of the structure of biomolecules and of how they transmit genetic information and store and use energy.
• Carbohydrates, lipids, proteins, and nucleic acids are the principal families of organic biomolecules. |
|
|
Explain monosaccharides.
|
Carbohydrates (saccharides), the most abundant of the organic biomolecules, are aldoses (polyhydroxyaldehydes) or ketoses (polyhydroxyketones) or compounds that can be hydrolyzed to aldoses or ketoses or are derived from them.
• Monosaccharides, the simplest saccharides, are the building blocks for producing larger saccharides, including disaccharides and polysaccharides. • Monosaccharides of biological interest are those from three to six carbons. • Monosaccharides are classified as aldoses or ketoses and by the number of carbon atoms in the chain containing the carbonyl group. • Aldoses of three or more carbons and ketoses of four or more carbons contain tetrahedral stereocenters. |
|
|
Continue to explain monosaccharides.
|
Almost all monosaccharides of biological interest belong to the D family, those having the D configuration (the -OH group on the right side in the Fischer projection) at the tetrahedral stereocenter farthest from
the carbonyl group. • D-Glucose, D-fructose, D-glyceraldehyde, dihydroxyacetone, and D- galactose are important monosaccharides. • D-Glucose is combined with D-fructose and D-galactose in sucrose and lactose, respectively. • Starch, glycogen, and cellulose are polymers of D-glucose. • A monosaccharide exists as cyclic hemiacetal α- and β-anomers in equilibrium with very low concentrations of the acyclic (open-chain) compound. |
|
|
What else should you know about monosaccharides.
|
Diastereomers are possible for monosaccharides containing more than one tetrahedral stereocenter. Anomers are diastereomers that differ in configuration only at the cyclic hemiacetal carbon.
• Monosaccharides undergo mutarotation, oxidation of the aldehyde group with Benedict’s reagent, and acetal formation. |
|
|
Explain the properties of disaccharides and polysaccharides.
|
• Disaccharides and polysaccharides are formed by glycosidic (acetal) linkages between monosaccharide residues.
• Disaccharides and polysaccharides are characterized by the monosaccharides from which they are produced, the positions of linkage between the monosaccharide rings, and the type of glycosidic linkage, α or β. Maltose, cellobiose, lactose, and sucrose are important disaccharides. • Starch (composed of amylose and amylopectin), glycogen, cellulose, and chitin are important polysaccharides. • Glycolipids and glycoproteins take part in cell-recognition processes. |
|
|
Explain photosynthesis.
|
• Photosynthesis, the process for carbon fixation, is the source of all carbohydrates.
• Plants use carbon dioxide, water, and sunlight to form monosaccharides, such as D-glucose, from which they synthesize starch, cellulose, and other saccharides, as well as lipids, proteins, and nucleic acids. • Animals are dependent on plants as their source of organic compounds and energy. |

