![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
7 Cards in this Set
- Front
- Back
|
Products of combustion reactions |
Cellular respiration: process when cells convert chemical energy stored in organic organic molecules (sugars) into usable energy
Reactants: Glucose C⁶H¹²O⁶ + O² gas
Products: CO² gas + H²O gas + ATP (energy) |
|
|
Combsution reactions |
Requires feul and oxygen 》releases energy (exothermic) Reactants: hydrocarbon (ethane, propane, methane, etc.) Products: CO² gas + H²o gas |
|
|
Complete combustions vs incomplete combustions |
Occurs when there's enough oxygen O² gas ??????? Occurs when there's not enough oxygen O² gas Both produce CO (Carbon monoxide) and CO² (Carbon dioxide) |
|
|
Complete combustion example |
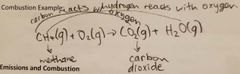
|
|
|
Emissions and combustion |
During most combustion reactions, the oxides of carbon, sulfur, and nitrogen (emissions) produced are released into the atmosphere |
|
|
Issues with combustion |
Metal atoms can be present in combustion emissions Examples: coal has lead and mercury Particulate matter (small solids suspended in the atmostphere) Example: soot, smoke, ash |
|
|
Carbon cycle |

|

