![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
13 Cards in this Set
- Front
- Back
|
Esters
What is the functional group of an ester? |
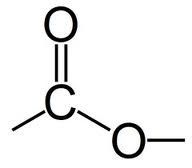
E |
|
|
Esters
How is an ester formed? |
From the reaction between a carboxylic acid and an alcohol in the presence of a metal catalyst.
Example - Propanoic acid + Ethanol -> Ethyl Propanoate + H2O |
|
|
Esters
How is an ester formed from acid anhydrides? |
Esters can be prepared by gently heating an acid anhydride with an alcohol.
This gives a better yeild.
Example - Ethanoic Anhydride + methanol -> Methyl Ethanoate + Ethanoic acid |
|
|
Esters
What is Esterification? |
The reaction of an alcohol with a carboxylic acid to form an ester and water. |
|
|
Esters
What is an acid anhydride? |
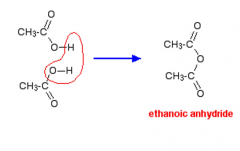
A molecule formed by the removal of a molecule of water from two carboxylic acids.a |
|
|
Esters
What is hydrolysis? |
A reaction with water or hydroxide ions that breaks a chemical compound into two compounds. |
|
|
Esters
Under what conditions can the hydrolysis of esters take place? |
With an aqueous acid or an aqueous alkali. |
|
|
Esters
What are the conditions for the acid hydrolysis of esters? |
The ester is heated under reflux with dilute sulfuric acid or dilute hydrochloric acid |
|
|
Esters
Draw the equation of the acid hydrolysis of propyl ethanoate. |

|
|
|
Esters
What are the conditions for an alkaline hydrolysis of an ester? |
Aqueous sodim or potassium hydroxide isrefluxed with the ester. |
|
|
Esters
Write the equation for the alkaline hydrolysis of ethyl propanoate. |
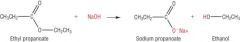
|
|
|
Esters
What is saponification? |
The reaction between an ester and aqueous sodium or potassium hydroxide, that is non-reversible, to produce the sodium salt of the carboxylic acid.
It is the basis of soap-making. |
|
|
Esters
What are the uses of esters? |
* Many esters are found in essential oils, obtained by steam distillation of organic plant matter.
Example - Benzyl ethanoate, CH3COOCH3C6H5, is found in many flowers and is the main component of essential oils from jasmine flowers. It is used for perfumery and cosmetics and apple and pear flavourings. It is found in shampoo, perfumes, fabric softner, soap, hairspray and deoderants.
Example - Oil of Wintergreen: the essential oil obtained from the Wintergreen plant. Can be massaged into muscles and joints to relieve pain as 'deep heat'. The ester involved is methyl salicylate. |

