![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
24 Cards in this Set
- Front
- Back
|
What three types of molecules are abundant in the extracellular matrix of all tissues?
|
1. Proteoglycans
2. Collagens 3. Multiadhesive matrix proteins |
|
|
What is the approximate split between fibrous and globular proteins in the human body?
|
Fibrous and globular proteins make up about half and half the total protein content in the body.
|
|
|
What are four key properties of fibrous proteins?
|
1. Extended protein structure
2. Insoluble in water (or lipid bilayers) 3. Secondary structure is simple based on one type 4. Quaternary structure is held together by covalent bridges |
|
|
What is the function of fibrous proteins?
|
Fibrous proteins are responsible for the structure of the body:
- keratin - external protection - toughness (hair, nails, outer skin) - elastin - in connective tissues - relates to elasticity-stretchability (ligaments, lung walls, aorta) - collagen - in connective tissues - relates to tensile strength (tendons, bones) - myosin - in muscle - contractile properties |
|
|
Where does α-keratin occur and what is it made by?
|
α-Keratin:
- occurs in hair, nails, outer layer of skin - almost the entire dry weight of these materials - made by epidermal cells |
|
|
What is the protein structure of α-Keratin?
|
Protein structure of α-keratin
- the entire secondary structure is α-helix - it is rich in amino acids that favour is α-helix formation (Phe, Ile, Val, Met, Ala) - these hydrophobic sidechains are on the α-helix surface - explaining its insolubility - also rich in Cys residues which form disulphide bridges between Cys in separate α-helices to connect them >Disulphide bridges occur in α-keratin - Disulphide bridges are also frequently used to stabilise the interior of a globular protein. - The more disulphides, the stronger the α-keratin |
|
|
What is the importance of disulphide bridges in α-keratin?
|
>Disulphide bridges occur in α-keratin
- Disulphide bridges are also frequently used to stabilise the interior of a globular protein. - The more disulphides, the stronger the α-keratin |
|
|
Describe the supersecondary structure of α-keratin.
|
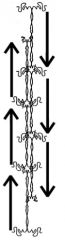
(“supersecondary” refers to all the secondary structures inside the structure).
- The association of long parallel α-helices gives α-keratin its toughness - The incorrect explanation of α-keratin structure still found in textbooks states that THREE α-helices supercoil around each other to form a protofibril, and that the association of 2 and 9 protofibrils forms a hair microfibril. >The up-to-date view is that TWO parallel α-helices supercoil around each other to form a dimer, then each dimer associates antiparallel with two other dimers in a staggered arrangement to form the protofibril. - The association of four protofibrils forms a four-stranded rope. - These successive overlaps explain why α-keratin is such a tough protein. |
|
|
What is elastin, where is it found and what makes it?
|
- major component of elastic fibres
- can stretch several times - then return to the original starting size - found in blood vessel walls (large arteries: the aorta), elastic ligaments, lung walls - made by fibroblasts and chondrocytes - implicated in cardiovascular disease and lung emphysema |
|
|
How is elastin formed?
|
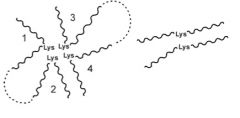
- synthesized as proelastin - converted to tropoelastin (mol wt 72000)
- crosslinking of tropoelastin via lysine residues gives elastin - crosslinks: either desmosine (4 Lys) linking 2, 3 or 4 molecules of tropoelastin, or a lysinonorleucine (2 Lys) or aldol link (2 Lys) linking 2 tropoelastin molecules - 2 lysines are crosslinked by oxidation (lysyl oxidase). In the following scheme, if both lysines are oxidised, an aldol link between the lysines is formed instead. - Desmosine is formed from 4 lysines, 3 of which are oxidised |
|
|
What is the amino acid composition of elastin?
|
33% Gly, 10% Pro, and Hyp, 23% Ala, 13% Val
- Hence 79% of the residues come from 4 amino acids. - There are large hydrophobic peptides rich in Ala, Val, Ile and Leu. - As these sidechains do not interact with each other by hydrogen bonds, their role appears to allow the elastin network to deform. |
|
|
What is the secondary structure of elastin?
|
A different type of helix structure from those in the -helix or the collagen triple helix is present. This is able to stretch and relax like a coiled spring. This is constructed from helices of -turns based on the sequence Val.Pro.Gly.Val (note the Gly residue: why is it there?), and is called the B-spiral
|
|
|
What is collagen and what is it synthesised by?
|
- The collagens are the most abundant proteins in the body
- Occur in connective tissues where tensile strength is needed - Examples: tendons, inner skin (72% collagen), cartilage (50% collagen), bones, cornea (68% collagen) - There are 30 different polypeptide chains; various combinations of these give rise to 16 different types of collagen - Example: Type I collagen in skin, bone and tendons has two α1 chains and one α2 chain. - Synthesized by fibroblasts and chondrocytes |
|
|
What are the four stages of collagen assembly?
|
(1) Synthesized as procollagen which is secreted from the cell (2) Cleaved to tropocollagen by procollagen peptidase
(3) Assembly of tropocollagen leads to the collagen fibre (4) Chemical crosslinking of tropocollagen strengthens the fibre |
|
|
Detail the first stage of collagen synthesis.
|
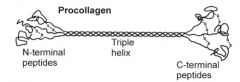
(First stage) Collagen synthesis: formation of procollagen The separate pro-α chains are synthesized inside the cell.
- Selected Pro and Lys residues are hydroxylated to hydroxyproline (Hyp) and hydroxylysine (Hyl) - Selected hydroxylysine residues are glycosylated - A typical single collagen sequence has about 900 residues, with an essential Gly residue at every third position and Pro and Hyp residues are common. - Hence the sequence is approximately (Pro.Hyp.Gly)300. (Related medical problem: osteogenesis imperfecta) - Three pro-α chains assemble at the C-terminus end starting with disulphide bridge formation between the 3 chains. The 3 chains then “zip” up to form procollagen. |
|
|
Detail the second stage of collagen synthesis.
|

(Second stage) Cleavage of procollagen to tropocollagen
- The N-terminal and C-terminal peptides of procollagen are cleaved by procollagen peptidase (Related medical problem: Ehlers-Danlos syndrome) - Tropocollagen is one of the most elongated proteins known: length 300 nm - Amino acid composition is 35% Gly, 21% Pro/Hyp, 12% Ala, and 32% for the rest - Secondary structure of tropocollagen is a triple helix |
|
|
What are the features of the collagen triple helix?
|
Features:
1. Three separate polypeptide chains arranged as a left-handed helix (note that an -helix is right-handed). 2. 3.3 residues per turn 3. Each chain forms hydrogen bonds with the other two. 4. Gly residues are essential at every third position in the sequence. The sidechain points towards the centre of the triple helix. (i) The Gly R group is only -H which enables the three polypeptide chains to be packed together; (ii) The Gly mainchain NH proton forms the key H-bond to the CO oxygen of the adjacent polypeptide. 5. Pro and Hyp residues are “imino acids”. The sidechain forms a covalent link with the mainchain N atom, and this ring structure stiffens the triple helix 6. Note that Gly residues are BURIED while the other two amino acids (including Pro and Hyp) are OUTSIDE the triple helix on its surface. The latter form inter-triple helix contacts to stabilize the collagen fibre. |
|
|
Describe the third stage of collagen assembly.
|
(Third stage) Assembly of tropocollagen
- Electron micrographs of a collagen fibre show periodic cross-striations with a repeat of about 67 nm. - This is accounted for by the formation of a one-quarter staggered array of tropocollagen molecules (i.e. each of which is displaced lengthwise by a quarter of its length). - There are 40 nm gaps between the tropocollagen molecules which is where calcium phosphate is deposited in bone formation. - This assembly forms spontaneously by means of noncovalent hydrogen bond interactions involving the OH group of hydroxyproline. (Related medical problem: scurvy) >Experiment: A synthetic polymer made up of Pro.Pro.Gly repeats forms a triple helix with a melting (unfolding) temperature of 24oC, which is below body temperature of 37C. The synthetic polymer made up of Pro.Hyp.Gly repeats has a melting temperature of 58C. |
|
|
Describe the fourth stage of collagen assembly.
|
>The collagen fibre is finally stabilised by covalent crosslinks that are formed between lysine residues.
>As for elastin, the action of lysyl oxidase generated the aldehyde form (CHO) of lysine (allysine) from the Lys residue. Joining one CHO and one NH2 group on two Lys residues leads to lysinonorleucine. >Joining two CHO groups leads to the aldol link. (Related medical problem: lathyrism) |
|
|
Name four collagen defects and disease.
|
1. Osteogenesis imperfecta
2. Ehlers-Danlos syndrome (type VII) 3. Scurvy 4. Lathyrism |
|
|
What is osteogenesis imperfecta?
|
>Osteogenesis imperfecta
- Results from a mutant collagen gene - a buried Gly residue is mutated to Cys. - The triple helix is partially unfolded at the N-terminal end The tropocollagen subunits cannot associate in a regular packing, and collagen fibre formation becomes weaker The clinical symptom is “brittle bones” and patients suffer from skeletal deformities |
|
|
What is Ehlers-Danlos (type VII)?
|
>Ehlers-Danlos syndrome (Type VII)
- Results from reduced levels of procollagen peptidase, so procollagen is not fully converted into tropocollagen - A high level of procollagen is found in the skin and tendons of patients - The 40 nm gaps between tropocollagen molecules become blocked by uncleaved peptides which prevent lysyl oxidase from acting on tropocollagen to create the crosslinks - Patients suffer from stretchable skin, hypermobile joints and short stature. |
|
|
What is scurvy?
|
>Scurvy - famous disease of medieval Europe.
- Hydroxyproline is important for the correct assembly of collagen fibres. - Hydroxyproline is only formed from Pro residues after procollagen has formed. - This reaction of Pro with prolyl hydroxylase requires ascorbate as a cofactor - otherwise known as Vitamin C - found in fresh fruit. The lack of Vitamin C in the diet leads to poor collagen fibril formation, so skin lesions develop and blood vessel walls are fragile. |
|
|
What is lathyrism?
|
>Lathyrism
- In the UK, lathyrism is typically an animal disease caused either by the ingestion of sweet pea seeds with α-aminopropionitrile or from a copper deficiency. - Alternatively it results from too low a level of lysyl oxidase (another form of Ehlers Danlos syndrome). α-aminopropionitrile prevents the conversion of Lys to the aldehyde form by irreversibly inhibiting lysyl oxidase. Note the similarity of this compound to the sidechain of a Lys residue. Lysyl oxidase requires copper for full activity, which is why a balanced diet is important for cattle and sheep. The lack of cross-link formation in collagen brings about lathyrism. |

