![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
What are the 5 most common cancers?
|
lung
stomach breast colon/rectum uterine cervix |
|
|
What is an oncogene?
|
a mutated or overly-expressed cellular gene that causes cancer
|
|
|
What is a proto-oncogene?
|
a normal cellular gene that can become an oncogene when mutated or when their expression is increased
|
|
|
What conversion causes cancer?
|
cancer occurs when proto-oncogenes are converted into oncogenes
|
|
|
Retroviruses and Cancer
|
Retroviruses use reverse transcriptase to convert RNA to DNA, which they insert into our genome.
They are involved in cancer genetics because sometimes when the viral sequences comes out of our genome it incorrectly excises one of our genes. |
|
|
How are Retroviruses incorporated into our genome?
|
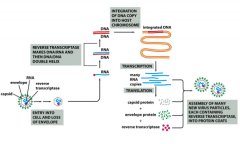
Retroviruses enter host cells already carrying reverse transcriptase and a cellular tRNA picked up from a former host cell, which is already base-paired to the viral RNA.
The tRNA facilitates immediate conversion of viral RNA to double-stranded DNA by the action of reverse transcriptase. Once converted to double-stranded DNA, the DNA enters the nucleus and is integrated into the host genome via a virally encoded integrate. *Retroviral DNA can integrate RANDOMLY or in a SITE SPECIFIC fashion. The method of integration is dependent on the particular retrovirus. * Mistakes in insertion can lead to mutations in genes flanking the region of integration. Once inserted into the host genome, the retroviral DNA can be transcribed and retroviral mRNA will be expressed. |
|
|
defective transducing retroviruses
|
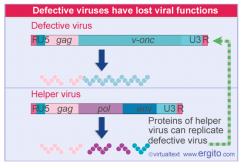
Retroviruses under rare circumstances can capture DNA from the host cell and incorporate it into their genome as RNA. If the captured DNA encodes for a proto-oncogene or tumor suppressor gene, the proto-oncogene/tumor suppressor gene can eventually be converted into an oncogene or an inactive suppressor gene via mutation.
Since the capsid of a retrovirus has limited space, the host DNA replaces retrovirus genes that are essential for producing a mature viral particle. Due to this, transforming viruses are generally defective, meaning that they do not contain the full complement of retroviral genes to produce a mature viral peptide. SO, they require confection of the cell with a Helper Virus, which will ultimately provide functional versions of the missing or nonfunctional genes. |
|
|
src: Rous Sarcoma Virus
|

has a complete(!) complement of all the retroviral genes necessary to produce a mature viral particle, in addition to the v-src (the src oncogene)
|
|
|
c-prefix
|
native proto-oncogene (a normal cellular gene that can become and oncogene due to mutations or increased expression) in the cell.
Example: c-src |
|
|
v-prefix
|
oncogene in a retrovirus.
Example: v-src |
|
|
Regulation of Src activity and its activation by an oncogenic mutation
|
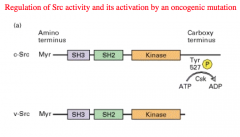
The Carboxy terminal of a normal Src gene expresses a Tyrosine residue, that when phosphorylated turns the enzyme activity OFF.
In the oncogene, this Tyrosine residue has been deleted and therefore there is no way to turn the enzyme off, and the Kinase gets overly expressed. |
|
|
Gain-of-Function Mutations
|
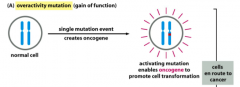
Turns a proto-oncogene into an oncogene.
Results in permanent activation of a gene. Requires at least 1 Mutation (oncogenes act in a dominant manner*: a gain-of-function mutation in a single copy of the cancer-critical gene can drive a cell toward cancer). Drives cell towards cancer. |
|
|
Loss-of-Function Mutations
|
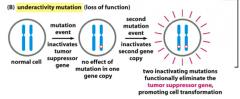
Drives cell towards cancer.
Leads to loss of function of a tumor suppressor gene, which in turn leads to activation of some other molecule because you can no longer suppress it. Inactivation of these genes leads to a cell that can no longer appropriately control cell division or apoptosis. Requires at least 2 mutations because mutations in tumor suppressor genes generally act in a recessive manner*: the function of both alleles of the cancer-critical gene must be lost to drive a cell toward cancer. Examples: -Inactivation of retinoblastoma protein. -Inactivation of p53 |
|
|
What types of accidents can convert a proto-oncogene to an oncogene?
|
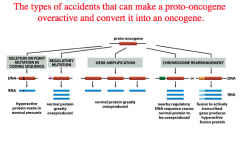
1. Deletion or point mutation in coding sequence.
-hyperactive protein made in normal amounts. 2. Regulatory mutation. -normal protein greatly over produced. 3. Gene amplification. -normal protein greatly overproduced. 4. Chromosomal rearrangement. -Nearby regulatory DNA sequence causes normal protein to be overproduced. -Fusion to actively transcribed gene produces a hyperactive fusion protein. |
|
|
What are some examples of Gain-of-Function Mutations?
|
1. A mutation which inactivates the GTPase activity of the G-alpha subunit will lead to the G-protein being constitutively active
-Even though we are losing GTPase activity, this would be a gain-in-function mutation. To determine the type of mutation look at the function and not just the actual mutation. 2. A point mutation in the transmembrane domain of a receptor that changes the conformation of the receptor permanently to the conformation the receptor would be in if a ligand was bound. 3. A mutation to the regulatory subunit of Protein Kinase A, which no longer allows the regulatory subunit to bind the catalytic subunit. |
|
|
what kind of proteins do oncogenes usually code for?
|
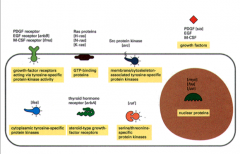
Most oncogenes code for proteins involved in growth and cell cycle pathways:
1. Growth Factors -can be over expressed or can bind and not dissociate from their receptors. 2. Growth Factor Receptors -Some have deleted extracellular domains, but the intracellular domain is constitutively active. 3. Intracellular Signal Transducers -Ras: is found mutated in 20% of all cancers 4. Nuclear Transcription Factors |
|
|
Ras
|
monomeric G-protein that is found mutated in 20% of all cancers.
Connects all of the growth factor receptors to MAP kinase cascades. When Ras is constitutively activated it is the same as if all of the growth factors receptors were active all the time. |
|
|
Warburg Effect
|
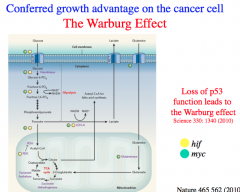
Cancer cells almost always exclusively utilize anaerobic respiration for energy production.
Due to the Warburg Effect, cancer cells export a great deal of lactic acid produced from fermentation to the extracellular environment and thereby generate a lower pH in the local environment that is supportive of their growth. Due to the Warburg Effect, cancer cells have large glucose requirements and DEPRIVE nearby healthy cells of glucose for growth. |
|
|
p53
|
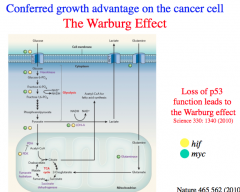
p53 suppresses the Warburg Effect.
p53 moderates glucose entry, promotes the pentose phosphate pathway, normalizes glycolytic activity, and increases pyruvate transport into the mitochondria. Therefore, *most cancers lead to a suppression of p53 and unsurprisingly, loss of p53 is a driver mutation. |
|
|
Germline Mutations
|
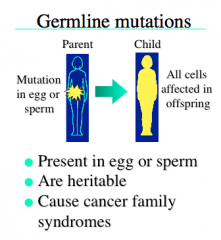
-Present in the egg or sperm
-are inherited -present in every cell |
|
|
Somatic Mutations
|
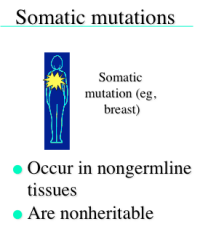
-occur in non-germline tissues
-are not inheritable -the number of somatic mutations we carry increases as we age. |
|
|
Driver Mutations
|
Give a growth advantage to the cell and directly cause cancer
|
|
|
Passenger Mutations
|
-Do not give a growth advantage, and do not contribute to cancer development.
-These mutations may play an important role in treatment and recurrence. -are somatic mutations present in cancer genomes |
|
|
early clonal expansion
|
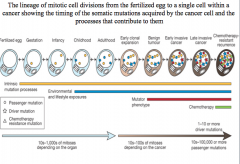
one driver mutation is insufficient for cancer development
|
|
|
benign tumor
|
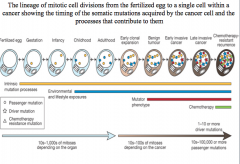
two driver mutations cause a benign tumor
|
|
|
early invasive cancer
|
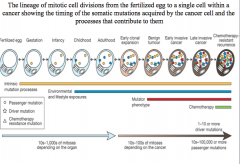
three driver mutations cause early invasive cancer
|
|
|
late invasive cancer
|
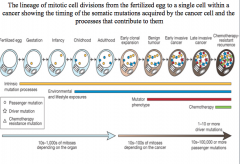
chemotherapy resistant genes result in late invasive cancer
|
|
|
Autosomal Dominant Inheritance
|
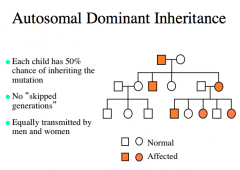
|
|
|
Autosomal Recessive Inheritance
|
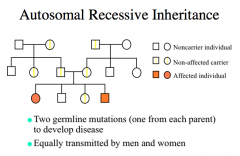
expression in phenotype may skip generations
|
|
|
Penetrance
|
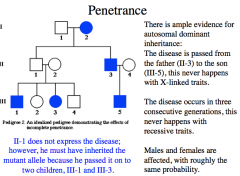
When the frequency of expression of the phenotype (when individuals have the mutant gene) is less than 100%, the gene is said to show incomplete penetrance*
Autosomal dominant disorders generally exhibit reduced penetrance* -most cancer susceptibility genes are dominant* with incomplete penetrance* Autosomal recessive disorders usually exhibit complete penitence. |
|
|
Factors Affecting Penetrance
|
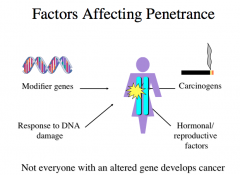
not everyone with an altered gene develops cancer because other factors play a role too.
|
|
|
Age-Specific Penetrance
|
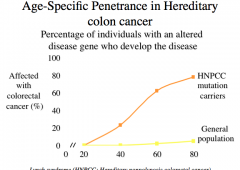
-penetrance exhibits age dependence
-as we get older, we collect mutations and at some point they reach a critical threshold that leads to cancer |
|
|
Expressivity
|
When severity of phenotype expression of a disorder differs in people with the same genotype, a gene is said to have "variable expressivity"
Example: Neurofibromatosis type 1 (NF1) |
|
|
How are most cancer genes inherited and expressed?
|
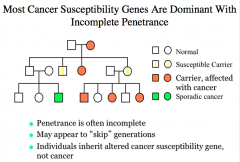
|
|
|
Two-Hit Hypothesis
|
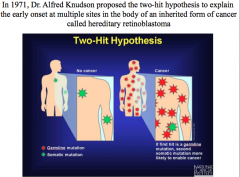
. Inheriting one germline copy of a damaged gene present in every cell in the body was not sufficient to enable this cancer to develop. A second hit (or loss) to the good copy in the gene pair could occur somatically, though, producing cancer. This hypothesis predicted that the chances for a germline mutation carrier to get a second somatic mutation at any of multiple sites in his/her body cells was much greater than the chances for a noncarrier to get two hits in the same cell.Tumor suppressors act recessive at the phenotypic level (both alleles must be mutated/lost for cancer to develop), but the "first hit" germline mutation at the genotypic level is actually inherited in an autosomal dominant fashion.
|
|
|
What other genetic factors can influence cancer development?
|
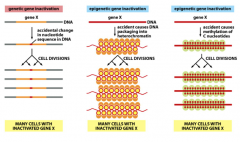
1. Mutation resulting from an irreversible change in DNA sequence
2. Epigenetics via dysfunctional nucleosome packaging. 3. Epigenetics via dysfunctional methylation patterns. |

