![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
81 Cards in this Set
- Front
- Back
|
What is the origin of the cells lining the mucosal surface (epithelium) of the intestine?
|
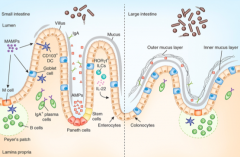
Crypt of Lieberkuhn stem cells
|
|
|
What cells are in the epithelium and overly the Peyer's Patches? Function?
|
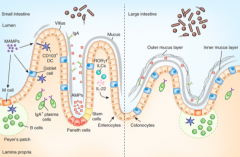
M cells - involved in antigen sampling and mucosal immunity
|
|
|
What kind of B cells are stimulated in the Peyer's Patches?
|
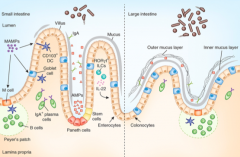
IgA producing, mature into plasma cells
|
|
|
How does the type and frequency of epithelial cells differ from the small to the large intestine?
|
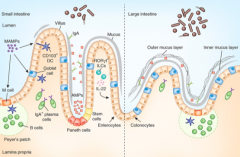
Large intestine has:
- More goblet cells - M cells - Outer mucus and inner mucus layer that is thick and continuous throughout (in small intestine it is thin and discontinuous) |
|
|
What is the intrinsic defense barrier in the GI tract?
|
Epithelium - in the intestine it is a single layer of cells that are hooked together with tight junctions and gap junctions
|
|
|
What are the types of extrinsic defense barriers in the GI tract?
|
- Mechanical / Involuntary Reflexes
- Structural - Chemical - Microbiological |
|
|
What are the mechanical / involuntary reflex extrinsic defense barriers in the GI tract?
|
- Cough
- Gag - Peristalsis (When these are lacking, you are more prone to infection) |
|
|
What are the structural extrinsic defense barriers in the GI tract?
|
Mucus
|
|
|
What are the chemical extrinsic defense barriers in the GI tract?
|
- Acid
- Enzymes - Antimicrobial peptides and polypeptides |
|
|
What are the microbiological extrinsic defense barriers in the GI tract?
|
Commensal microbiota (specifically in intestines)
|
|
|
What is the organization of the intestinal mucus?
|
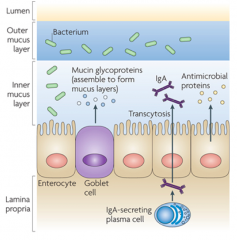
- In large intestine this barrier is continuous, in small intestine it is discontinuous
- Made of outer and inner mucus layers - Bacteria usually reside in the outer mucus layer - A lot less bacteria in the inner mucus layer where it is secreted by the Goblet Cells - IgA is also found in the inner mucus layer which is secreted by plasma cells in the lamina propria to keep it relatively sterile - Antimicrobial proteins also released into inner mucus layer |
|
|
What is mucus made of?
|
Mucins - viscoelastic gel
|
|
|
What are mucins secreted by? Characteristics of secretion?
|
Specialized Goblet cells
- Secretion can be continuous or regulated - 10L released / day |
|
|
What is the function of Mucins?
|
- Forms selectively permeable mucus blanket
- Mucus-commensal interactions - Bacterial exclusion - Containment of secreted antibodies and antimicrobials |
|
|
What are the contents of mucus?
|
- Mucins
- Water - Ions - Proteins - Lipids - Antibodies - Antimicrobial peptides - Bacteria |
|
|
How do mucins interact with commensal organisms?
|
- Specific binding of some commensals via adhesions allows bacteria to "graze" on mucus
- Bacteria cleave specific sugars from tips of oligosaccharides - Small subset of commensals digest mucus |
|
|
How do mucins exclude bacteria? How do they interact?
|
- Thickness and viscosity contribute to exclude bacteria
- Bacteria and LPS have been shown to induce MUC gene expression and secretion - Pathogens have developed specific mechanisms to evade the barrier (flagella, interference w/ exocytosis) |
|
|
How do mucins function in containment of antibodies and antimicrobials?
|
- IgA and other secreted antibodies bind mucus through low affinity bonds and interact with commensals and pathogens
- Cationic antimicrobial peptides may be contained via electrostatic interactions with mucins |
|
|
Where do the secreted chemical defenses come from?
|
- Mostly from epithelium
- Some inflammatory cells and bacteria also contribute |
|
|
What are the secreted chemical defenses?
|
- Acid (stomach)
- Lectins / collectins (lung surfactant proteins) - Enzymes and inhibitors - Antimicrobial peptides and proteins |
|
|
What enzymes and inhibitors are secreted to defend against pathogens?
|
- Lysozyme (muramidase, hydrolyze peptidoglycans)
- Peroxidases (MPO) - SLPI (protease inhibitor) - sPLA2 |
|
|
How do secreted antimicrobial peptides and proteins function to protect against pathogens?
|
- Direct killing by forming pores in microbial cell walls and membranes (eg, defensins (alpha, beta, and theta), cathelicidins)
- Iron sequestration (lactoferrin, lipocalin) prevents bacterial growth since they require iron (some bacteria have mechanisms to extract iron) |
|
|
What kind of cells secrete antimicrobial peptides?
|
- WBCs
- Epithelial cells lining mucosal surface |
|
|
What is the action of the antimicrobial peptides secreted by WBCs and epithelial cells?
|
- Broad-spectrum of activity
- Make holes in bacterial cell membranes |
|
|
What is an example of a cationic antimicrobial peptide found in mammals? Characteristics?
|
Defensins:
- Invariant 6-cysteine array involved in intramolecular disulfide - α-defensin (neutrophils and paneth cells) and β-defensin (mucosal epithelium) types - |
|
|
Where are α-defensins expressed? When are they expressed?
|
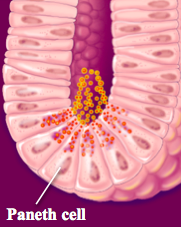
- Neutrophils
- Paneth cells - Expressed constitutively (always) |
|
|
Where are β-defensins expressed? When are they expressed?
|
- Mucosal epithelium
- Constitutive and inducible expression |
|
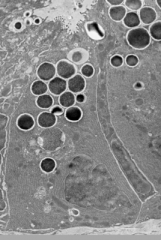
What are the contents of Paneth Cell granules?
|
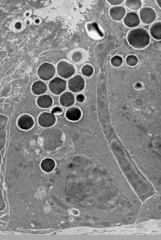
- α-Defensins (HD5 and HD6)
- CRS peptides - Lysozyme - sPLA2 - RegIII-γ - Angiogenin-4 - α-1-Antitrypsin - TNF-α - IL17a - MMP7 - IgA |
|
|
What are the forms of Defensin found in Paneth cells?
|
- HD5
- HD6 |
|
|
What are the serological secreted immunological defenses?
|
- sIgA - predominant Ig
- IgM - IgG - IgE - IgD |
|
|
Which is the predominant immunoglobulin in mucosal secretions?
|
sIgA (secretory IgA)
|
|
|
What are the components of IgA? Function?
|
- Secretory component (SC) is part of pIgA (polymeric, mucosal) and transports IgA into secretions
- Alpha chain - J chain (only associated with pIgA) |
|
|
If someone has an IgA deficiency (1 in 800), what happens?
|
Usually doesn't cause significant problems because they compensating by inducing release of IgM
|
|
|
What are the limitations of IgM as an immunological defense?
|
- Secretory component (SC) transports IgM into secretions
- May not be transported as well as IgA because of MW restrictions in SC dependent transport |
|
|
What are the relative levels of IgG in immunological defenses?
|
- Found at same levels as IgM
- Proportion of IgA to IgG varies by site and time of collection (ie, proportion varies through menstrual cycle) |
|
|
Where/when is IgE found?
|
- Found in low concentration
- Associated with mucosal allergic responses |
|
|
Where/when is IgD found?
|
- Found in low concentration
- Found in milk and saliva |
|
|
What are the two structures of IgA? How do they differ?
|
- Serum IgA - predominantly monomeric
- Mucosal IgA - predominantly polymeric |
|
|
How is IgA synthesized?
|
Synthesized as monomer and forms pIgA (polymeric) prior to secretion
|
|
|
How much IgA is secreted daily? How is it removed?
|
- 4g secreted daily
- Metabolized and cleared by liver |
|
|
How do IgA secreting plasma cells get induced to secrete IgA? Effect of IgA?
|
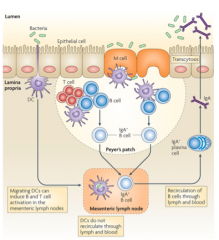
- Bacteria at mucosal surface (commensal and pathogenic)
- Dendritic cells sample bacteria by sticking out an arm which can then present this to B cells - M cells in epithelium also sample bacteria and activate plasma cells - Plasma cells release IgA (non-inflammatory) which binds to bacteria in lumen and prevent them from gaining access to epithelium |
|
|
What are the biological activities of IgA?
|
- Inhibits adherence of bacteria to epithelium (repels attachment to mucosa)
- Mucus-trapping (traps microbes in mucus) - Virus neutralization (inhibits attachment) - Enzyme and toxin neutralization - Inhibits antigen penetration (including food) |
|
|
How does IgA inhibit enzymes and toxins? Which ones specifically by location?
|
- Saliva: inhibits enzymes from oral bacteria (neuraminidase, hyaluronidase, chondroitin sulfatase, glucosyltransferase)
- Gut: neutralizes bacterial toxins (cholera toxin, heat-labile enterotoxin, clostridial enterotoxin A) |
|
|
What are the mechanisms by which microbes evade the action of IgA?
|
- Specific IgA proteases
- Other proteases - Glycosidases - IgA binding proteins |
|
|
What is the action of specific IgA proteases for microbial evasion of IgA?
|
- Cleaves one of several prolyl-seryl or prolyl-threonyl peptide bonds in hinge region
- Cleaves off intact Fab fragments that retain binding activity - Exquisitively substrate specific, not inhibited by protease inhibitors - Causes local IgA deficiency in vivo |
|
|
What microbes are associated with having specific IgA proteases to evade the anti-microbial action of IgA?
|
Meningitis: H. influenzae, N. meningitidis, S. pneumoniae
|
|
|
What is the action of other proteases for microbial evasion of IgA?
|
Wide spectrum protease can cleave IgA
|
|
|
What microbes are associated with having wide spectrum proteases used to evade the anti-microbial action of IgA?
|
- Peridontal pathogen: Porphyromonas gingivalis
- Some intestinal Enterobacteriaceae |
|
|
What is the action of glycosidases for microbial evasion of IgA?
|
IgA is heavily glycosylated and thus subject to damage by bacterial glycosidases, which disrupt the conformation, net charge, and resistance to proteolysis
|
|
|
What is the action of IgA binding proteins for microbial evasion of IgA?
|
- Cell surface proteins bind IgA non-specifically (ie, Fc region)
- Lectin binding of O-linked carbohydrate in IgA hinge region |
|
|
An elderly nursing home patient with dementia presents with fever and elevated white blood cell count. A chest x-ray suggests lobar pneumonia. Which is the MOST LIKELY mechanism of pathogenesis?
a) Decreased secretion of mucus b) IgA deficiency c) Loss of gag/cough reflex d) Disruption of the commensal microbiota secondary to poor nutrition e) Reduced stomach acid secondary to protein pump inhibitor use |
Loss of gag / cough reflex
|
|
|
What is a microbiome?
|
Totality of native microbes, their genetic information, and the milieu in which they interact
|
|
|
What are the three major classes of bacteria in a healthy microbiota?
|
- Symbionts
- Commensals - Pathobionts |
|
|
What is the action of symbionts?
|
Share mutual relationship with the host, have known health promoting factors
|
|
|
What is the action of commensals?
|
Permanent residents of this ecosystem and provide no benefit or detriment to the host
|
|
|
What is the action of pathobionts?
|
Live as commensals (providing no benefit or detriment) but have the potential to induce pathology
|
|
|
What is the term for an altered microbial composition? What are the implications of this state?
|
Dysbiosis - associated with diseases like IBD, auto-immunity, obesity, diabetes, asthma and allergy, colorectal carcinoma, etc
|
|
|
What is the role of the mucosal surface of the GI tract? Characteristics?
|
- Constantly in contact with microbes
- Primary role is to allow normal physiological function while protecting from infection - Mucosal immune system protects host from microbiota, but microbiota has a symbiotic role in host protection and host physiology |
|
|
How does the composition of microbes in the lower GI tract (small and large intestines) compare?
|
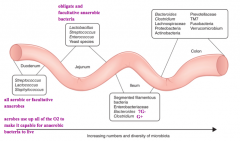
- Microbial composition changes from proximal to distal intestine, moving from domination by aerobic species to facultative and obligate anaerobes in colon
- Also as you go more distally there are increasing numbers and diversity of microbes |
|
|
What factors shape the microbial composition?
|
- Immune factors: defensins, IgA
- Non-Immune factors: O2 tension, pH, digestive enzymes, bile salts, mucus, DIET |
|
|
How does breast milk help select for the microbial composition of the GI tract?
|
- Human milk contains HMO (human milk oligosaccharides)
- HMOs are completely indigestable by humans but can be digested by Bifidobacterium - Bifidobacterium has major host benefits for infants |
|
|
What are the main functions of the commensal intestinal microbiota?
|
- Protection
- Structure - Metabolism |
|
|
How do the commensal intestinal microbiota supply protection to the intestine?
|
- Pathogen displacement
- Nutrient competition - Receptor competition - Produce anti-microbial factors (eg, bacteriocins, lactic acids) |
|
|
What are the structural functions that the commensal intestinal microbiota provide to the intestine?
|
- Barrier fortification
- Induction of IgA - Apical tightening of tight junctions - Develops immune system |
|
|
What are the metabolic functions that the commensal intestinal microbiota provide to the intestine?
|
- Control IEC diferentiation and proliferation
- Metabolize dietary carcinogens - Synthesize vitamins (eg, biotin and folate) - Ferment non-digestable dietary residue and endogenous epithelial-derived mucus - Ion absorption - Salvage of energy |
|
|
What happens to mice that lack a mature mucosal immune system?
|
- Underdevelopment of lymphatic tissues
- Delayed B cell migration in response to bacterial antigen - Reduced antibody diversity - Reduced lymphocyte responsiveness |
|
|
What happens to mice that lack a mature mucosal immune system when they develop a normal mucosal immune system?
|
- Increased lymphocyte infiltration of gut mucosa
- Germinal center formation in Peyer's patches - Induction of innate antimicrobial effector molecules - Treatment with bacterial polysaccharide from bacterial symbiont (B. fragilis) restores many immune functions |
|
|
How do commensal microbiota prevent pathogen colonization?
|
• Bacteriocin production
• SCFA production • Consumption of oxygen • Competition for nutrients • Competition for attachment sites • Induction of epithelial antimicrobials • Induction of mucus production and secretion |
|
|
How do commensal bacteria regulate digestion?
|
- Mediation of bile acid synthesis
- Lipid absorption - Amino acid metabolism - Vitamin synthesis, such as Vitamin K - SCFA production - Byproducts of commensal fermentation (metabolites) regulate the immune system |
|
|
Which is a function of the intestinal microbiota?
a) Water absorption b) Digestion of dietary sucrose c) Displacement of bacterial pathogens d) Synthesis of vitamin C e) Production of human milk oligosaccharides |
Displacement of bacterial pathogens
|
|
|
What diseases are associated with the intestinal microbiome?
|
- Inflammatory Bowel Disease
- Obesity and obesity-related disease (diabetes and non-alcoholic fatty liver disease) - Cancer - Allergy / asthma |
|
|
How is the intestinal microbiome associated with Inflammatory Bowel Disease?
|
Abnormal host immune response to the colonizing bacteria in a genetically susceptible host leads to uncontrolled inflammation
|
|
|
How is the intestinal microbiome associated with Obesity and obesity-related diseases (Diabetes and Non-Alcoholic Fatty Liver Disease)?
|
Efficiency of bacterial fermentation and products of metabolic byproducts can contribute to obesity and its complications
|
|
|
How is the intestinal microbiome associated with cancer?
|
Byproducts of bacterial metabolism can promote cell growth and act as carcinogens
|
|
|
How is the intestinal microbiome associated with allergies and asthma?
|
- "Hygiene hypothesis" - decreased early infections lead to immune dysregulation
- "Microflora hypothesis" - dysbiosis leads to immune dysregulation - "Vanishing microbiota hypothesis" - interactions w/ certain microbes are wired into our immunoregulatory networks based on constant presence in our environment, resulting in tolerance; loss of these co-evolved microbes can result in allergic hyper-responsiveness |
|
|
What happens in Inflammatory Bowel Disease?
|
Abnormal bacterial colonization (dysbiosis) and abnormal immune function leads to abnormal host response
|
|
|
What is the definition of a probiotic?
|
A viable microbial food supplement which beneficially influences the health of the host
|
|
|
What are the criteria for a probiotic?
|
– Origin of microbe
– Stability in the GI tract (acid and bile resistant) – Viability – Adherence to human intestinal mucus/mucosa – Antimicrobial activity against pathogens |
|
|
What are the functions of a probiotic?
|
– Improves intestinal barrier function
– Stimulates mucin secretion – Stimulates antimicrobial peptide expression – Inhibits adherence and invasion of pathogens – Enhances IgA production – In epithelial cell culture, some probiotics have anti-inflammatory activity (attenuate IL-8 and TNF-α secretion, inhibit NF-κΒ pathway, prevent apoptosis) – Metabolic and neurologic effects are currently being examined |
|
|
What can probiotics be used to treat? How?
|
Treatment of immune disorders
- Restore the barrier function (prevent excess antigen transfer across skin and gut barriers) - Skew T cell immune responses to Th1 type |
|
|
What are probiotics being studied to treat?
|
- Pouchitis
- IBS: Irritable Bowel Syndrome - Cow's milk allergy - Atopic excema (in high risk neonates) - Allergy |

