![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
46 Cards in this Set
- Front
- Back
- 3rd side (hint)
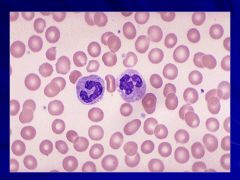
|
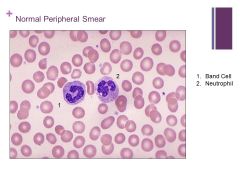
Peripheral Smear Caption: Caption: (WBC Lab)
|
|
|
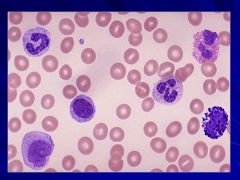
|
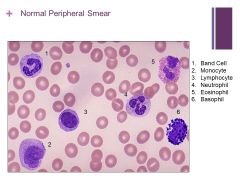
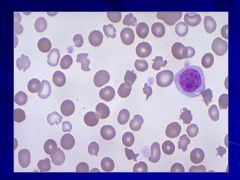
Normal smear that happens to have all the cells that could be in the smear Caption:
|
|
|
|
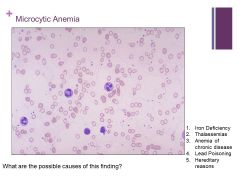
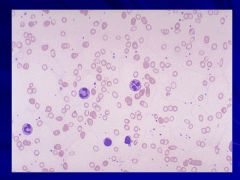
MCV <70fL! Caption: |
|
|
|
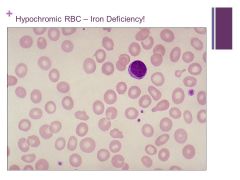
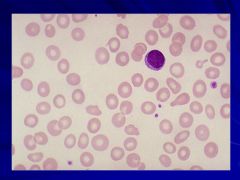
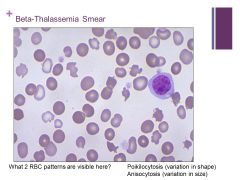
Hypochromic cells – most common cause is iron deficiency Caption:
|
|
|
|
|
|
|
|
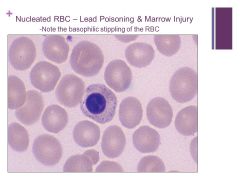
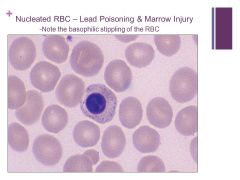
Note that we can know this is an RBC and not a WBC because the plasma color is so similar to the RBC – the nucleus just wasn’t ejected yet. Caption: |
|
|
|
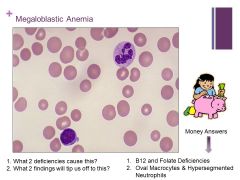
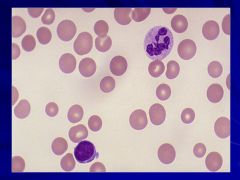
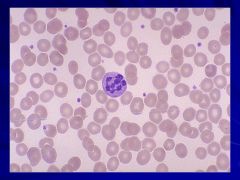
Macrocytic anemia – MCV >100fL Caption: |
|
|
|
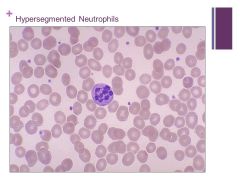
Always think megaloblastic anemia and B12 or folate deficiencies!! Caption:
|
|
|

|
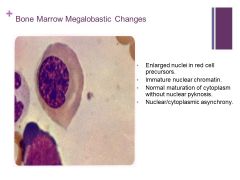
Pyknosis – irreversible condensation of chromatin in a cell undergoing apoptosis or necrosis |
|
|
|
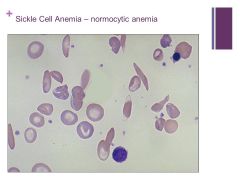
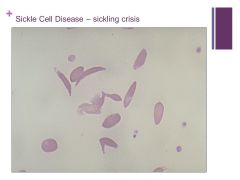
SS is homozygous for sickle cell and you get anisocytosis Caption: |
|
|
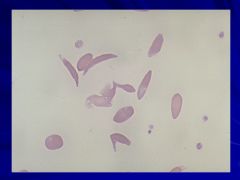
|
|
|
|
|
|

|
|
|
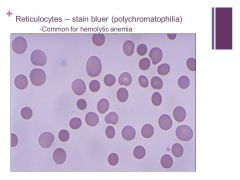
|
|
|
|
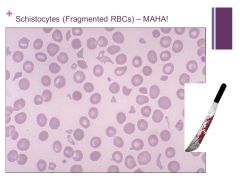
MAHA = microangiopathic hemolytic anemia – schistocytes are indicative of this or other intravascular hemolysis (Found with TTP – ADAMST13 deficiency and vWF aggregation) Caption: |
|
|
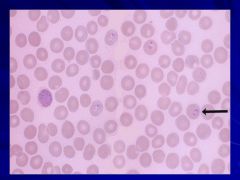
|
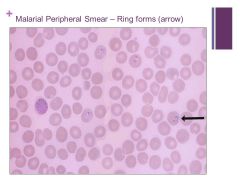
Can get into endothelial cells, can effect the liver Caption:
|
|
|
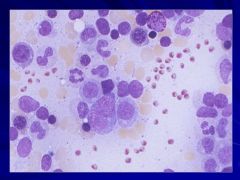
|
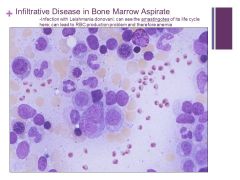
Can see yellow fat cells and different cell lines of the marrow but the little red things are not normal! Caption: |
|
|
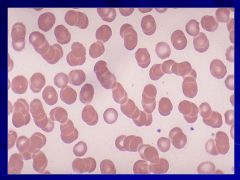
|
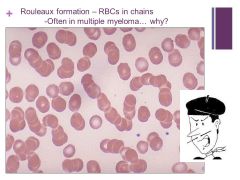
Happens with increased serum proteins in the blood i.e. fibrinogen or globulins Caption:
|
|
|
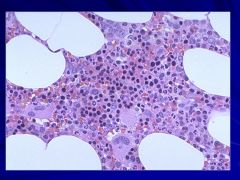
|
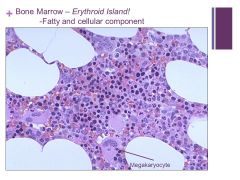
Erythroid precursors are very dark – can’t tell the difference in a bone marrow specimen really unless you do a bone marrow aspirate smear Caption: |
|
|
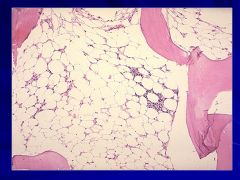
|
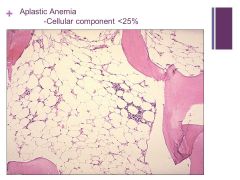
Aplastic Anemia Caption:
|
|
|
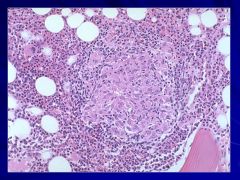
|
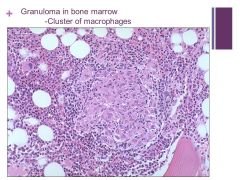
May be present in terms of infection (fungal or TB), CT disorders, sarcoidosis, or some types of lymphoma Caption: |
|
|
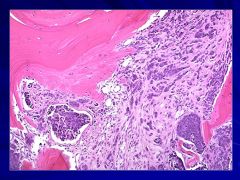
|
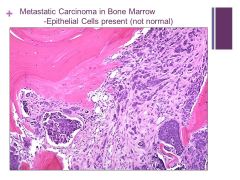
Dark blue clusters are replacing the normal hematopoeitic cells Caption:
|
|
|
|
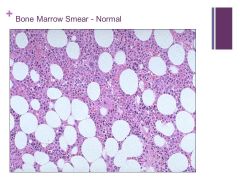
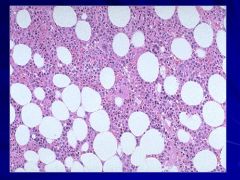
When looking at bone marrow look first at cellularity (ratio of fat to cells - at age 50 it should be 50:50) – Need Patient’s Age! Caption: |
|
|
|
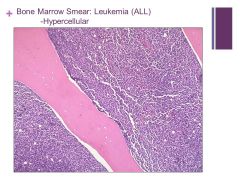
Almost 100% cellularity – will never be normal even in an infant Caption: |
|
|
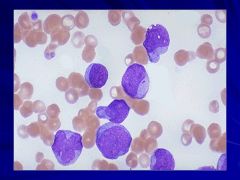
|
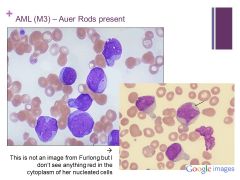
Can decide it’s myeloid instead of lymphoid because myeloid cells make granules and we “see” Auer Rods Caption: |

|
|
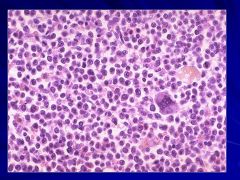
|
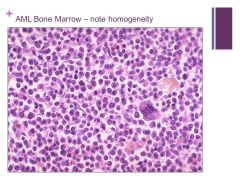
Myeloproliferative disorders Caption:
|
|
|
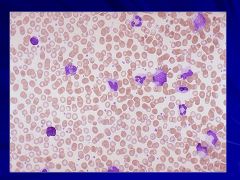
|
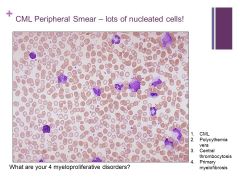
Lone megakaryocyte in marrow Caption: |
|
|
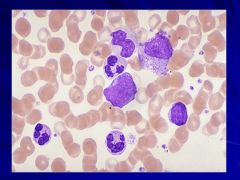
|
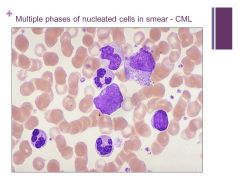
NOTE: there are not many BLASTS here so you can safely say it is chronic not acute Caption:
|
|
|
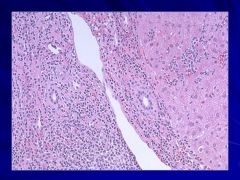
|
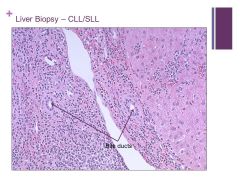
Very small lymphocytes (SLL) Caption: |
|
|
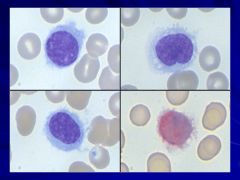
|
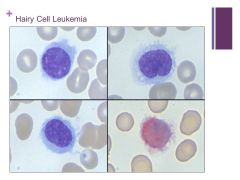
More common in men than women (older patients usually) often present with splenomegaly Caption:
|
|
|
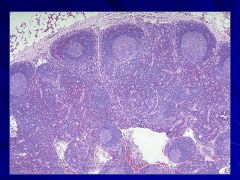
|
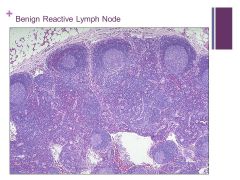
-Adipose tissue at the top (perinodal) right above the capsule Caption: |
|
|
|
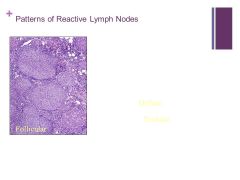
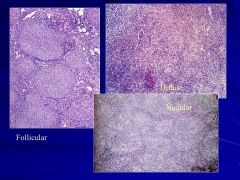
FOLLICULAR PATTERN: follicular lymphoma Caption: Not on website? |
|
|
|
They are very large but we can’t really tell it’s lymphoma from the gross picture Caption:
|
|
|
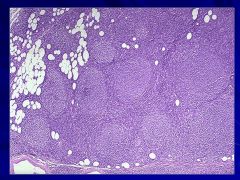
|
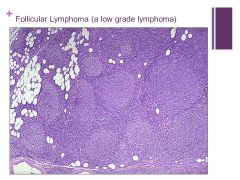
-Follicular pattern (follicular lymphoma) – low grade Caption: |
|
|
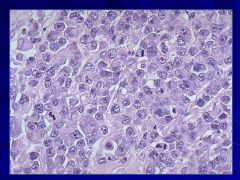
|
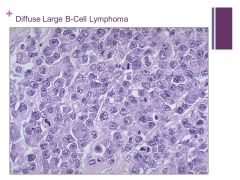
Can occur in all age groups Caption: |
|
|
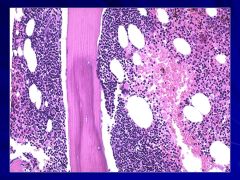
? |
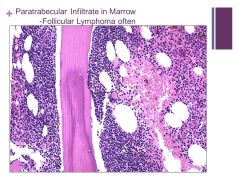
Often associated with follicular lymphoma Caption:
|
|
|
|
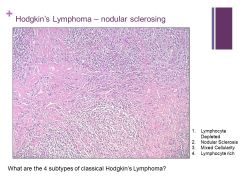
Bands of fibrosis are present! Caption: |
|
|
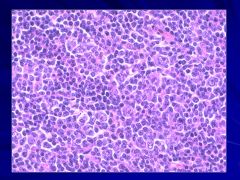
|
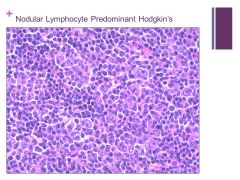
Nodular pattern Caption: |
|
|
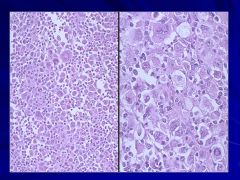
|
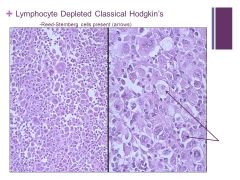
Hard to tell this is lymphocytic at all (cells are different) Caption: |
|
|
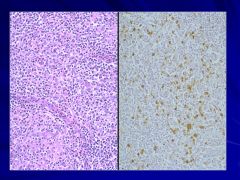
|
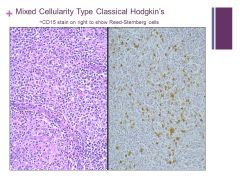
Mixed cellularity in background macrophages, small lymphocytes, plasma cells, eosinophils Caption:
|
|
|
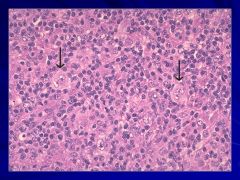
|
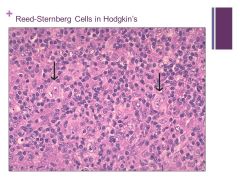
Binucleate cells that are indicative of Hogdkin’s Caption: |
|
|
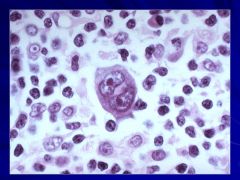
|
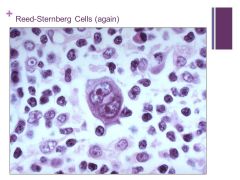
|
|
|

|

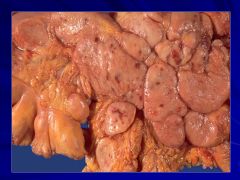
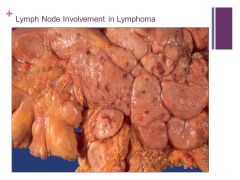
If liver is involved it puts the cancer at a higher stage Caption:
|
|
|
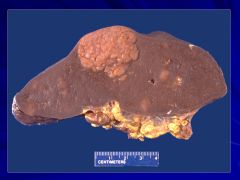
|
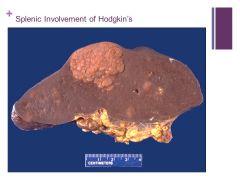
**Hematopoietic diseases are much more likely to produce splenic masses than mets Caption: |
|
|
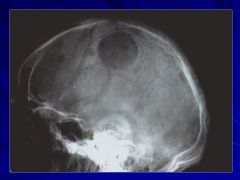
|
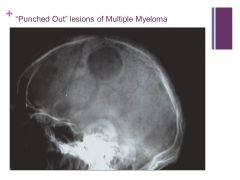
No this is not histology. Caption: |
|
|
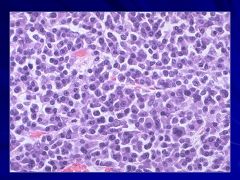
|
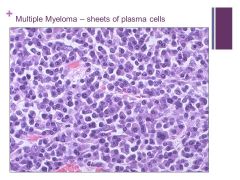
Progressive disease Caption: |
|
|
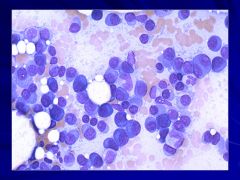
|
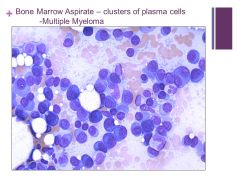
|
|

