![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
191 Cards in this Set
- Front
- Back
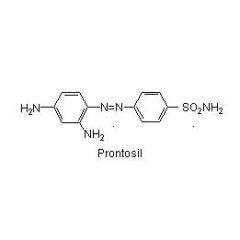
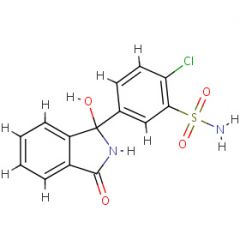
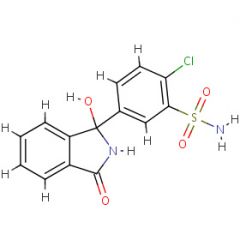
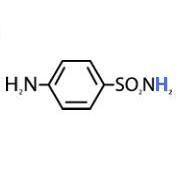
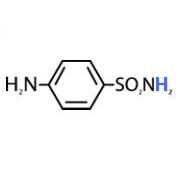
Prontosil is ____ against bacteria in vitro but ____ in vivo.
____ is the active form. |
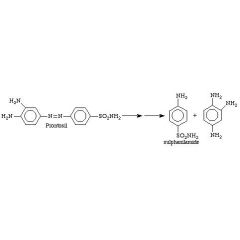
Prontosil is inactive against bacteria in vitro but active in vivo.
Sulfanilamide |
|
Generic:
TC: |
Generic: Hydrochlorothiazide
TC: Diuretic |
|
Generic:
TC: |
Generic: Chlorthalidone
TC: Diuretic |
|
Generic:
TC: |
Generic: furosemide
TC: diuretic |
|
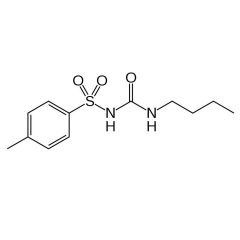
Generic:
TC: |
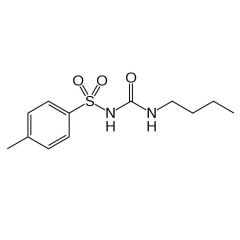
Generic: tolbutamide
TC: oral hypoglycemic |
|
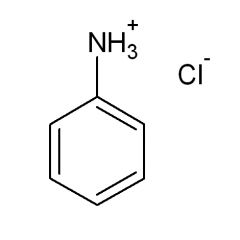
The pKa of the conjugate acid of aniline is:
|
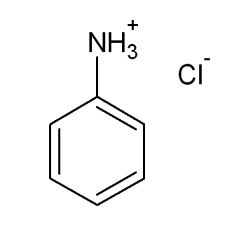
4.6
|
|
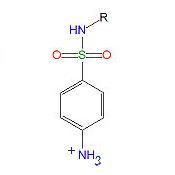
The pKa of the anlinium ion is lowered from 4.6 to ____ by the addition of the para SO2NHR
|
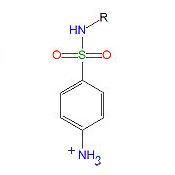
2 - 3
|
|
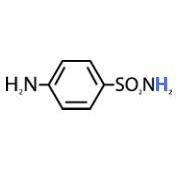
The pKa of sulfanilamide is:
|
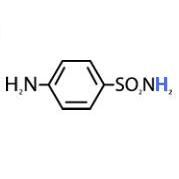
10.4
|
|
Draw the most stable form of sulfanilamide (N1 proton removed)
|
Neg charge on nitrogen unstable unless it can be dist to other sides (ex sulfisoxazole)
|
|
Why do the sulfonamides below have pKa values 3 - 5 orders of magnitude lower than sulfonamide?
|
Heterocyclic aromatic ring attached to N1 is electron withdrawing, lowers pKa of N1 proton
|
|
|
Phenyl rings are not strong enough e- withdrawing groups to sig lower pKa. ____ rings will. Stronger e- wd groups (NO2) have been tried but were too toxic.
|
Heterocyclic
|
|
|
Sulfanilamide causes severe kidney damage due to ____ because:
A. B. |
crystallization
A. Neither the parent sulfonamide or acetylated metabolite is very water soluble B. both sulfonamides and their metabolites (mostly N4 acetylated) are excreted in urine. |
|
|
If urine pH < Pka for a sulfonamide, ____ salt form exists and solubility is ____ because sulfonamides are ____.
|
If urine pH < Pka for a sulfonamide, little salt form exists and solubility is low because sulfonamides are acidic.
|
|
Label the carbons and nitrogens on sulfanilamide
|
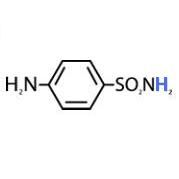
|
|
|
Give 4 methods of increasing the amount of sulfonamide dissolved in urine
|
1. increase urine flow - drinking enough fluids
2. Raise urine pH 3. Use sulfonamides with lower pKa values 4. Mix sulfonamides - solubilities are independent of one another (ex triple sulfas) |
|
|
Folate coenzymes are essential to life in bacteria, plants, animals, and humans. They provide ____ carbon units for synthesis of a number of essential biomolecules.
|
one.
|
|
|
Bacteria ____ use folic acid from the host (won't cross cell wall). Sulfonamide and sulfone antibacterials act as competitive inhibitors of ____ incorporation into folic acid - specifically inhibiting the enzyme ____ ____
|
can't
PABA - para-aminobenzoic acid dihydropteroate synthatase |
|
|
Bacteria and protozoa biosynthesize ____ ____, however, humans and other animals must get folic acid from their diet.
|
dihydrofolic acid
|
|
|
Answer if humans, bacteria, or both have enzymes: give inhibitors of each
Dihydropteroate synthatase: Dihydrofolate reductase: Dihydrofolate synthase: |
Dihydropteroate synthatase: bacteria, sulfonamides, sulfones
Dihydrofolate reductase: both, trimethoprim (selective for bact) Dihydrofolate synthase: bacteria |
|
|
Trimethoprim is a ____ ____ inhibitor with ____ times greater selectivity for the bacterial form of the enzyme than for the human form.
|
Trimethoprim is a dihydrofolate reductase inhibitor with 100000 times greater selectivity for the bacterial form of the enzyme than for the human form.
|
|
|
Trimethoprim is ____ - ____ times more potent than sulfamethoxazole. The optimum ratio of these combined 2 agents is equal to the ratio of the ____ of each acting independently.
|
Trimethoprim is 20 - 100 times more potent than sulfamethoxazole. The optimum ratio of these combined 2 agents is equal to the ratio of the MIC (min inhibitory conc) of each acting independently.
|
|
|
Optimal ratio of (given pharmacokinetic differences) sulfamethoxazole to Trimethoprim:
|
10 : 2
|
|
|
Trimethoprim inhibits bacterial ____ ____, preventing reduction of ____ to ____.
|
Trimethoprim inhibits bacterial dihydrofolate reductase, preventing reduction of FH2 to FH4.
|
|
|
Sulfonamides are considered ____ rather than ____.
|
Sulfonamides are considered bacteriostatic rather than bacteriocidal.
|
|
|
Bacterial resistance to sulfonamides mechanisms.
1. 2. 3. 4. 5. |
Bacterial resistance to sulfonamides, mechanisms.
1. increase PABA production 2. alter binding strength to pathway enzymes 3. decrease cell membrane permeability 4. active efflux of sulfonamide 5. R-factor (plasmid) transfer of resistance to prev non-resistant |
|
|
SAR - sulfonamides
Modification of the N4 aniline amino group results in ____ of ____, although prodrugs can be prepared. |
SAR - sulfonamides
Modification of the N4 aniline amino group results in loss of activity, although prodrugs can be prepared. |
|
|
SAR - sulfonamides
Exchange of the SO2-NH for SO2-C6H4-p-NH2 (a ____) retains antibacterial activity. Exchange for CO-NH2 or CO-C6H4-p-NH2 ____ ____ activity. |
SAR - sulfonamides
Exchange of the SO2-NH for SO2-C6H4-p-NH2 (a sulfone) retains antibacterial activity. Exchange for CO-NH2 or CO-C6H4-p-NH2 greatly decreases activity. |
|
|
SAR - sulfonamides
N1 substitution with e- wd group ____ activity and ____ pharmacokinetics. N1 disubstitution generally ____ activity. |
SAR - sulfonamides
N1 substitution with e- wd group increases activity and improves pharmacokinetics. N1 di-substitution generally abolishes activity. |
|
|
Max antibacterial effect is seen in sulfonamides with pKa = ____ - ____
Typical pH of urine = ____ |
Max antibacterial effect is seen in sulfonamides with pKa = 6.7 - 7.4
Typical pH of urine = 6 |
|
|
hypersensitivity reaction seen with sulfonamides (other sulfa drugs too) causing skin eruptions, allergic myocarditis, photosensitization:
|
Stevens-Johnson Syndrome
|
|
|
Hematologic rxns with sulfas.
1. 2. 3. |
1. agranulocytosis
2. aplastic anemias 3. hemolytic anemias (pats with G6P dehydrogenase deficiency) |
|
|
Seen in patients with inadequate fluid intake
|
crystalluria
|
|
|
Most sulfonamides tend to be ____ absorbed and ____ ____.
Exceptions: 1. 2. |
Most sulfonamides tend to be quickly absorbed and well distributed.
Exceptions: 1. topicals for burns 2. agents used in ulcerative colitis and to reduce bowel flora |
|
|
N4-acetate metabolites of sulfonamides have ____ lipid solubility, thus greater protein binding than the parent drug. Yet, they are more rapidly ____ than the parent.
|
increased, excreted
|
|
|
two sulfonamide metabolites:
1. 2. |
two sulfonamide metabolites:
1. N4 acetates (inactive) 2. N4 glucuronides (inactive - D-glucose attached via B linkage) |
|
|
Name sulfonamide antibacterial agent without heterocyclic on N1
|
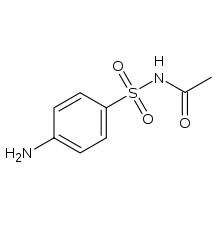
sulfacetamide
|
|
|
Name 2 sulfonamides used for Ophthalmic infections
|
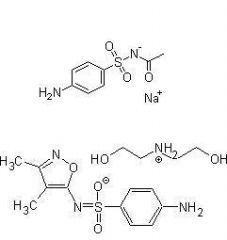
Sulfacetamide sodium and Sulfisoxazole diolamine
|
|
|
list two sulfonamides for burn therapy
|
1. silver sulfadiazine
2. mafenide acetate |
|
|
Name nonabsorbable NSAID sulfonamide for IBD
|
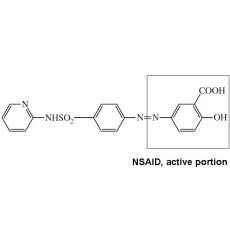
Sulfasalazine: NSAID
|
|
|
Tetracyclines are ____ antibiotics
|
broad spectrum
|
|
|
list tetracyclines still on the market
|
chlortetracycline, demeclocycline, doxycycline, methacycline, minocycline, oxytetracycline, tetracycline
|
|
|
tigecycline is considered a "glycylcycline", but it is in fact a ____.
|
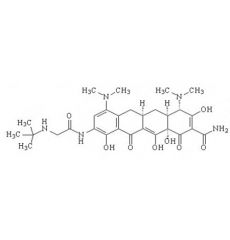
tetracycline
|
|
|
Tetracyclines can be obtained by a fermentation process with ____ ____.
|
Streptomyces sp.
|
|
|
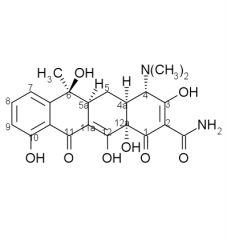
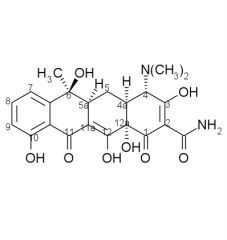
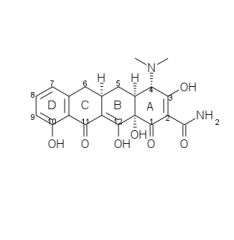
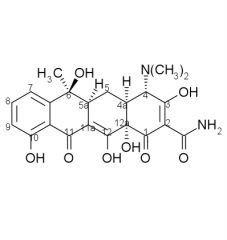
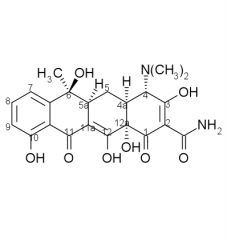
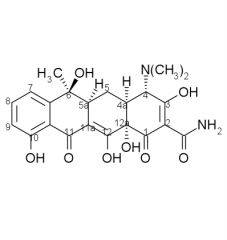
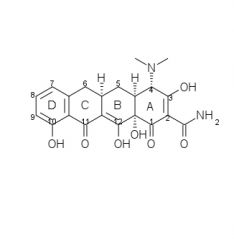
Draw the structure and number carbons of tetracycline
|
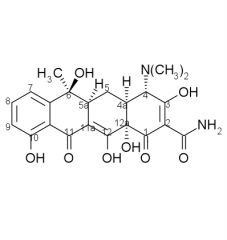
*6a & 10a - no potential for substitution
|
|
|
____ and ____ both have a 5alpha-OH group
|
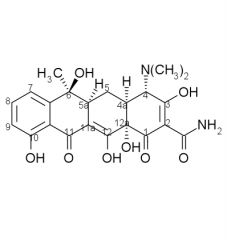
oxytetracycline, doxycycline
|
|
|
Acidity of protons alpha to carbonyls
|
.
|
|
|
KOH or NaOH may be used to make salts of tetracyclines, but they are ____.
HCL salts are the most common and are encapsulated because of their bitter taste. HCL will mask the fishy smell/taste of the ____ group. |
unstable, amine
|
|
|
Tetracyclines are ____, salts will form with either acids or bases. They exist as ____ in neutral solutions.
|
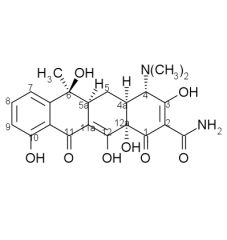
amphoteric, zwitterions
|
|
|
Solutions of tetracycline HCL need to be stabilized with excess ____ to prevent the from crystalizing out of solution.
|
acid
|
|
|
In solutions of intermediate pH (4-8), tetracyclines can undergo ____ at ____ to produce compounds known as ____, which have about 5% of the activity of their natural form. This is ____ catalyzed.
|
epimerization, C4, epitetracyclines, base
|
|
|
Tetracycline epimerization: under acidic conditions, equilibrium with equals amounts of each isomer is established in ____.
|
one day
|
|
|
Tetracycline epimerization:
At neutral pH, the epi form of tetracycline may account for up to ____ of the mixture. |
90%
|
|
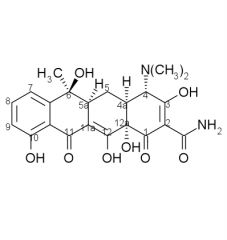
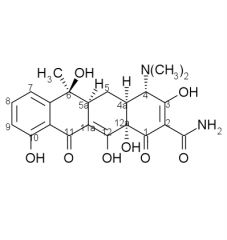
Tetracycline stability:
Tetracyclines having a ____ at ____ are attacked by both acids and bases causing loss of activity by modifying the ____ ring. |
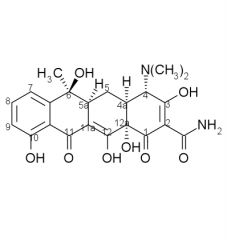
beta-OH, C6, C
|
|
|
Acid Catalyzed Tetracycline Degradation forms a ____ like system known as a ____. The tetracycline must have a ____ on ____ for this to occur.
|
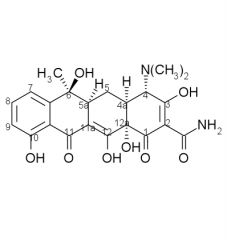
naphthylene, anhydrotetracycline, beta-OH, C6
|
|
|
Acid catalyzed breakdown products of tetracycline include:
A. B. C. |
A. 4-epitetracycline
B. anhydrotetracycline - must have beta-OH at C6 C. anhydro-4-epitetracycline - must have beta-OH at C6 |
|
|
Tetracycline: Basic conditions promote a reaction between the ____ ____ and the ____ ____, this cleaves the bind between ____ and ____ giving a ____. Product is inactive.
|
Tetracycline: Basic conditions promote a reaction between the C6 OH and the C11 carbonyl, this cleaves the bind between C11 and C11a giving a lactone. Product is inactive.
|
|
|
Tetracyclines form stable chelates with many ____ and ____ ____. These chelates are water ____ and ____ tetracycline absorption. Thus, they shouldn't be taken with ____, ____, ____, ____ ect.
|
Tetracyclines form stable chelates with many di- and trivalent metals. These chelates are water insoluble and impair tetracycline absorption. Thus, they shouldn't be taken with milk, antacids, hematinics, ect.
|
|
|
Tetracycline affinity for Ca++ causes them to be deposited in newly formed ____ and ____ as ____ ____ complexes. Keep away from:
|
bones, teeth, tetracycline-calcium orthophosphate
Keep away from: young children and nursing mothers |
|
|
Mech of action of Tetracyclines:
|
Inhibit bacterial protein synthesis by binding to the 30S ribosomal unit and prevent aminoacyl tRNA from binding to the mRNA-ribosome complex during translation. Both aminoacyl tRNA and TCN require Mg++ to bind the ribosome.
|
|
|
Resistance to tetracyclines results from:
A. B. C. D. |
A. loss of ability to actively transport TCNs.
B. Presence of TCN-binding proteins at cell surface, C. Synthesis of bacterial proteins which associate with the ribosomes to allow protein synthesis to continue in presence of bound TCNs. D. R factor (plasmid) mediated active efflux of TCN-Mg++ complexes. |
|
|
Tetracyclines have the ____ spectrum of any known antibacterial agents. They are active against ____ and ____ bacteria, ____, ____, ____, ____
|
gram (-), gram (+), spirochetes, mycoplasmas, Rickettsiae, Chlamydia
|
|
|
Tetracyclines are ____, thus are not best choice in life threatening infections.
|
bacteriostatic
|
|
|
Tetracyclines are incompletely absorbed, thus, if the natural bacterial flora are sensitive, there may be danger of a ____ ____ from ____ ____.
|
super infection, Candida albicans
|
|
|
____ due to resistant ____ ____ and ____ ____ may develop when TCNs are used for a long time.
|
Superinfections, Staphylococcus aureus, Pseudomonas aeruginosa
|
|
|
Tetracycline SAR:
1. Derivatives with fewer than ____ rings are inactive or nearly inactive. 2. Simplest active derivative with broad spectrum activity is ____. The integrity of substituents at (list) can not be drastically changed without great decrease in activity. |
1. 4
2. 6-demethyl-6-deoxytetracycline, 1, 2, 3, 4, 10, 11, 11a, 12 |
|
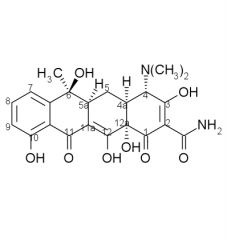
Tetracycline SAR cont:
3. A-ring a. ___ ___ (C1-C3) must be intact b. replacement of the ___ with other fx groups abolishes activity c. ___ of the amide decreases activity d. dimethyl amino at C4 must be present and have ___ |
a. enolized tricarbonylmethane
b. amide c. monoalkylation d. alpha-stereochemistry |
|
Tetracycline SAR cont:
4. ______ ring fusion is necessary. 5. alkylation at ___ abolishes activity, enolization of the C11-C12 beta-diketone is crucial to activity. 6. ___ of all ring junctions important. Epimerization of ___ decreases activity. Dehydration of ___ decreases activity due to ___ ___. |
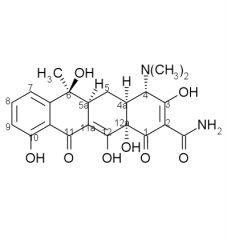
4. cis A/B
5. 11a 6. stereochemistry, 5a, 5a - 6, ring aromatization |
|
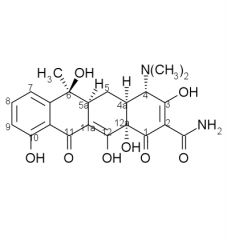
Tetracycline SAR cont:
7. Esters of the ___ OH group are inactive except for formyl because it ___ easily. 8. Substituents at ___, ___, ___, ___, ___ are less sensitive and may increase activity. |
7. 12a, hydrolyzes
8. 5, 5a, 6, 7, 9 |
|
|
Tetracycline SAR cont:
9. Acid stable 6-deoxy-TCNs and 6-demethyl-deoxy-TCNs have been used to prepare many mono/di-substituted derivatives at C7 and C9 *effects at C8 not investedgated, no directors to that position |
|
|
|
Tetracycline SAR cont:
10. Neither ___ or ___ is necessary for activity. *___ don't form anhydro-TCNs under acidic conditions and they don't undergo lactonization under basic conditions. |
10. 6alpha-CH3, 6-beta-OH
*6-deoxy-derivatives |
|
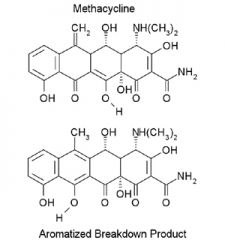
C-ring Aromatization of Methacycline
(acid catalyzed). Methacycline is stable, but aromatization does occur. Draw the mechanism. |
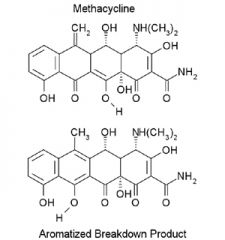
The final product is inactive.
|
|
|
Removal of the 6-OH increases the ___ ___.
|
*finish slide after clarification on slide 30 on Vd effects
|
|
|
Pharmacokinetics:
Passive ___ ___ ___ and higher fraction of ___ ___ contribute to lower renal clearance and prolonged duration of action of doxycycline and minocycline. The more lipid soluble TCNs distribute better into ___ ___ tissue. |
renal tubular reabsorption, protein binding, poorly vascularized
|
|
|
Bacterial cell wall function:
1. 2. 3. |
1. Provide semipermeable barrier with environment through which only desirable substances can pass.
2. Protects from changes in osmotic pressure. 3. Prevents digestion by host enzymes. |
|
|
The cell wall of gram-positive bacteria:
Outside: Peptidoglycan layer: |
Outside: carbohydrates and proteins make up antigen determinants - adherence to target cells.
Peptidoglycan layer: N-acetylglucosamine and N-acetymuramic acid sugars linked via B(1,4) glycosidic linkages. Lactic acid moiety on acetylmuramic acid is linked to series of amino acids which form cross-links between strands. |
|
|
Attached to the NAM (N-acetylmuramic acid) lactic acid moeity is a series of amino acids: ___-___-___-___-(___ before cross-linking).
The ___ stereochemistry is believed to protect the peptidoglycan from hydrolysis by host enzymes. |
L-ala-D-glu-L-lys-D-ala-(D-ala)
"D" |
|
|
Cross-linking steps Gram positive cell wall (btw 2 strands)
1. Serine OH on ___ ___ ___ reacts with the ___ D-ala of strand A displacing the ___ D-ala to afford an ___. 2. The terminal ___ of strand B then reacts with the ___ ___ formed btw D-ala on strand A and CWT, releasing CWT and cell wall in linked via a ___ bond. |
Cross-linking steps Gram positive cell wall (btw 2 strands)
1. Serine OH on cell wall transamidase reacts with the penultimate D-ala of strand A displacing the terminal D-ala to afford an ester. 2. The terminal Gly of strand B then reacts with the ester carbonyl formed btw D-ala on strand A and CWT, releasing CWT and cell wall is linked via a peptide bond. |
|
|
Cell wall transamidase (CWT) is also a ___ ___ ___. Cross-linking is very sensitive to ___ antibiotics. The beta-lactam receptors are also ___ ___ ___. There are at least ___ types of PBP.
|
Cell wall transamidase (CWT) is also a penicillin binding protein (PBP). Cross-linking is very sensitive to beta-lactam antibiotics. The beta-lactam receptors are also penicillin binding proteins (PBP). There are at least 7 types of PBP in gram + organisms..
|
|
|
Binding of beta-lactam (penicillin) antibiotics to:
PBP-1 (transpeptidase) leads to: PBP-2 (transpeptidase) leads to: PBP-3 (transpeptidase) leads to: PBP-4-6 (carboxypeptidases) leads to: Only a small % of penicillin G binds to PBP 1-4. Most binds 5 & 6 |
PBP-1 (transpeptidase) leads to: cell lysis
PBP-2 (transpeptidase) leads to: oval cells lacking rigidity and inhibits division PBP-3 (transpeptidase) leads to: abnormally long filamentous shapes & failure to produce septum PBP-4-6 (carboxypeptidases) leads to: no lethal effects Only a small % of penicillin G binds to PBP 1-4. Most binds 5 & 6 |
|
|
Gram + bacteria secrete the enzyme ___ outside the cell. Thus, it must be replenished frequently.
|
beta-lactamase
|
|
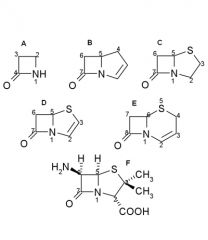
Penicillin nomenclature:
Name these B - lactams |
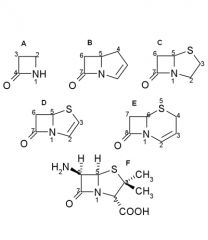
A.Monobactam
B.Carbapenem C.Penam D.Penem E.Cefem F.6-Aminopenicillanic acid |
|
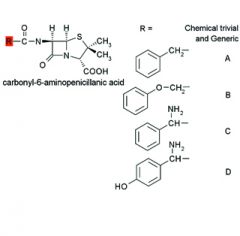
|
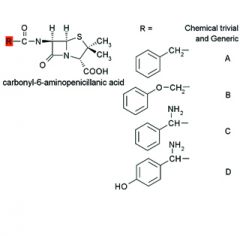
A. Benzylpenicillin
Penicillin G B. Phenoxymethylpenicillin Penicillin V C. D-alpha-Aminobenzylpenicillin Ampicillin D. D-alpha-Amino- p-hydroxybenzylpenicillin Amoxicillin |
|
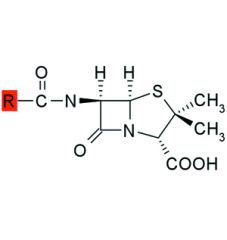
The bond between the carbonyl carbon and nitrogen of a beta-lactam ring is highly strained and thus reactive.
Hydrolysis in aq solution may be retarded by storing penicillins between pH ___ & ___ and by ___ of the solution. |
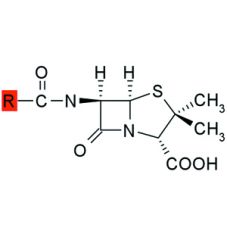
6, 7, refrigeration
|
|
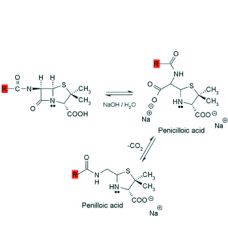
Alkaline hydrolysis of penicillins:
1. Rapid and gives ___ products. 2. Product is ___ which decarboxylates to ___. 3. Penicilloic acid is also the product formed when the bacterial enzyme ___ hydrolyzes penicillin. |
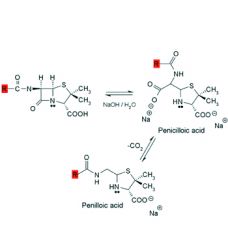
1. inactive
2. penicilloic acid, penilloic acid 3. beta-lactamase |
|
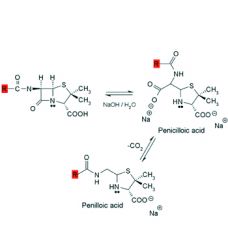
Alcohols and amines will also cleave the beta-lactam ring to afford esters and amides respectively.
Acid catalyzed hydrolysis is more complex and affords ___, ___, and ___. Analogues with ___ ___ groups attached to the side chain carbonyl exhibit some resistance to acid catalyzed degradation. |
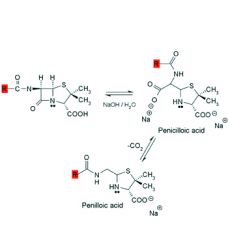
1. penicillamine, penilloaldehyde, penilloic acid
2. electron withdrawing |
|
|
Beta-Lactam Protein Binding:
Serum protein binding for the penicillins is: 1. directly related to the ___ of the side chain, as it increases the serum protein binding increases. 2. inversely related to the degree of ___ of the side chain. As degree increases, serum protein binding decreases. |
1. lipophilicity
2. ionization |
|
|
Only ___ penicillin is active, thus protein binding may decrease the bactericidal concentration of the drug. However, the degree of protein binding has little effect on the ___ or ___ of action.
|
free, half-life, duration
|
|
|
Penicillins are secreted into renal tubular fluid by an ___ ___ ___ in the ___ convoluted tubule of the kidney. (organic acid (anion) transport system, OATS)
|
anion secretion system, proximal
|
|
|
Mechanism of cross-linking (detailed)
Starting with D-ala-D-ala segment, show how the serine residue on CWT (PBP) catalyzes the attachment of the penultimate D-ala to the end of the pentaglycl chain. |
*CWT (PBP) is regenerated at end.
|
|
|
Mechanism of the beta-lactams:
Show mechanism of beta-lactam complexing with CWT (PBP) to disable it. |
*CWT (PBP) is NOT regenerated - effect is bactericidal.
|
|
|
The geometry of a beta-lactam antibiotic closely resembles that of ___ and CWT mistakenly accepts this as substrate. CWT is ___ ___ reactive towards a beta-lactam that to it's own substrate due to the ___ ___ in the beta-lactam.
|
D-ala-D-ala, much more, ring strain
|
|
|
Acylated enzyme formed with a Beta-lactam doesn't resemble the normal acylated-CWT, thus:
1. 2. |
1. crosslinking does not occur
2. CWT is not regenerated |
|
|
The unatural stereochemistry of the D-ala-D-ala residues protect the bacterial cell wall from hydrolysis by ___ enzymes. The selectivity of CWT for D-amino acids protects ___ cells from its actions.
|
host, host
|
|
|
Resistance to beta-lactamases:
A. Intrinsic: 1. decreased cellular ___. 2. PBP with lower binding affinity - ex: ___ B. Elaboration of beta-lactamases (R-factor mediated) - these are ___ proteases that react with the beta-lactam bond before it can reach CWT. |
1. uptake
2. methacillin resistant Staphylococcus aureus B. serine |
|
|
A ___ of beta-lactamase can destroy a ___ amount of beta-lactam antibiotic.
|
small, large
|
|
|
Resistance to Beta-lactams:
Gram-___ bacteria continuously shed beta-lactamase to the outside of the cell to react with beta-lactams. Gram-___ bacteria restrict the beta-lactamase to the ___ space. This reduces the need for ___. |
positive, negative, periplasmic, resynthesis
|
|
|
Bulky side chain groups on beta-lactams confer ___ ___.
|
beta-lactamse.
ex: methacillin |
|
|
___ to ___ % of the population is allergic to beta-lactam antibiotics. Drug rash or itching may occur - occasionally - profound CV collapse, shock, & possibly death
|
6 - 8
|
|
|
Beta-lactam allergy is a ___ reaction, host proteins rxn with beta-lactams generates the antigen.
|
haptenic
|
|
|
Beta-lactam spectrum: primarily gram-positive.
Exception: ___ and ___ have broader spectrum than most of the other penicillins. |
Neisseria gonorrhea - gram neg.
Ampicillin, amoxicillin |
|
|
Draw penicillin G - give trivial chem name:
Useful against: |
benzylpenicillin
Useful against: nonresistant Group A & Group B beta-hemolytic Strep, Pneumococcal pneumoniae, H. influenza, Strep. pneumoniae, Strep. pyogenes |
|
|
Draw penicillin V - give trivial chem name:
More ___ stable that G, it can be taken ___. Similar spectrum to G but ___ ___. Gives higher more prolonged ___ ___ than G. Electron withdrawing groups on side chain ___ acid resistance. |
phenoxymethylpenicillin
acid, orally less potent blood levels increase |
|
|
Draw methicillin.
DF: ___ ___ near side chain amide bond ___ sensitivity to beta-lactamase. Both ___ positions need to be substituted. |
DF: IV
steric hinderance, decreases ortho |
|
|
Methicillin use:
1. Only used for ___ producing ___ ___. 2. The increased incidence of MRSA is due to: 3. Methicillin is an efficient inducer of ___. Therefore it is used only in infections that uniquely require it. Very acid ___, only used ___. |
1. beta-lactamase, Staph. auerus.
2. reduction in the affinity of PBP for penicillins in resistant strains. 3. beta-lactamase, labile, IV |
|
|
Orally active beta-lactamse resistant penicillins:
Draw isoxazoylpenicillins. 1. ___ potent against gram-positive bact that don't produce beta-lactamase (Staph and Strep), but show activity against ___ producing bact. 2. Acid ___, thus ___ active. ___ % protein bound, thus not a good choice for ___. |
1. less, beta-lactamase
2. stable, orally, 90, septicemia |
|
|
Beta-lactamase sensitive broad spectrum Oral penicillins:
Draw ampicillin. 1. improved spectrum, including some ___ organisms. 2. ___ ___ increases acid stability, thus orally active. 3. Hydrolyzed by beta-lactamase - increased resistance seen. 4. Penicillin derivative most associated with drug induced ___. 5. Can ___ to give high molecular weight aggregates that are antigenic. |
1. gram-negative.
2. protonated amine 4. rash 5. self-condense |
|
|
Beta-lactamase sensitive oral penicillins:
Draw amoxicillin. 1. ___ oral absorption relative to ampicillin 2. ___ drug induced diarrhea relative to ampicillin 3. dosed ___ while ampicillin is ___ |
1. increased
2. decreased 3. tid, qid |
|
|
Beta-lactamase sensitive oral penicillins:
Carbenicillin: 1. side chain COOH increase the ___ activity. 2. One of the broadest spectrum penicillins, but use restricted to ___ ___ (high dose tx), ___ ___ (high dose tx), ___ & ___ ___. 3. Only dosage form in US is ___. |
1. gram-negative
2. Pseudomonas aerogenosa, Proteus vulgaris, Enterobacter, d. Serratia 3. tablet (sodium salt) |
|
|
Carbencillin Decarboxylation:
Readily decarboxylates to afford ___ ___. Which has no activity against the organisms for which Carbenicillin is indicated. |
penicillin G
|
|
|
Beta-lactamase inhibitors:
Class I: ___ ___, ___ and ___. These are ___ ___ enzyme inhibitors which irreversibly inhibit the enzyme. Class II: ___ Possess broad-spectrum antimicrobial activity and also inhibit beta-lactamase - mech based also. Used mainly for antimicrobial activity. |
Class I: Clavulanic acid, Sulbactam, Tazobactam
Mechanism based Class II: Carbapenems |
|
|
Class I - Clavulanic acid
Draw the mechism for beta-lactamase inhibition. 1. It is acid ___ because it lacks amide side chain at C6. 2. It has ___ ___ antibacterial activity. |
*Irreversible inhibitor
1. stable 2. weak intrinsic |
|
|
Class I beta-lactamase inhibitor - Sulbactam
A penicillanic acid ___ |
sulfone
|
|
|
Class I beta-lactamase inhibitor - Tazobactam
More potent than ___ |
Sulbactam
|
|
|
Class II (Carbapenems) beta-lactamase inhibitors - Thienamycin
1. Subject to ___ ___ by the cystenamide side chain when in solution. |
1. intermolecular aminolysis
|
|
|
Class II (Carbapenems) beta-lactamase inhibitors - Imipenem
*broadest antimicrobial spectrum off all beta-lactam antibiotics. When used to treat UTI, imipenem is hydrolyzed by ___ ___, an enzyme located in the ___ ___ of the kidneys. Cilastitin sodium is added because it is a renal ___ inhibitor. |
renal dehydropeptidase-I, brush border, dehydropeptidase-I
|
|
|
first cephalosporin discovered
|
Cephalosporin C
Not potent enough to be a useful antibiotic. |
|
|
Cephalosporin chemistry:
1. Chephalosporins are ___. 2. draw basic Chephalosporin structure. |
1. bactericidal
2. |
|
|
1. Allergic rxns towards to cephalosporins are:
2. Patients who have had rapid or severe allergic rxns to penicillins should: |
1. less common and less severe
2. NOT be given cephalosporins |
|
|
First gen Cephalosporins primarily active against:
|
gram-positive bacteria
|
|
|
1. Second gen cephalosporins are active against:
2. They also exhibit ___ activity. |
1. gram-positive, some gram-negatives (H. influenzae)
2. antianaerobic |
|
|
1. Third gen cephalosporins are less active against:
2. but much more active against ___ than either first or second gen. 3. frequently used to treat: |
1. Staph
2. gram-negative bacteria 3. multidrug-resistant nosocomial (hospital infection) infections |
|
|
1. Fourth gen cephalosporins have a spectrum like ___ ___:
2. Also more active against ___ organisms. |
1. 3rd gen plus others resistant to 3rd gen
2. gram-positive |
|
|
Chephalosporins:
Because of the involvement of the ___ leaving groups, active participation by the ___ side chain in hydrolysis (like at C6 in penicillins) is ___ ___. |
C3, C7, rarely invoked
|
|
|
Cephalosporins possessing a tetrazolethiomethyl leaving group have been associated with:
A. B. C. Tetrazolethiomethyl group is found in: |
A. hemorrhagic clotting problems
B. Acute alcohol intolerance in some patients due to inhibition of aldehyde dehydrogenase C. cefamandole, cefmetazole, cefotetan, cefoperazone |
|
|
Metabolism of Cephalosporins:
1. Hydrolysis of the C3 acetyl ester gives an ___. 2. Once hydrolyzed, a ___ can form. 3. Show this mechanism. 4. Remember - penicillin binding proteins an absolute requirement for a free ___ group to mimic the terminal COOH of ___. |
1. alcohol - greatly decreases activity.
2. lactone 3. see figure 4. carboxyl, D-ala-D-ala |
|
|
Quinolones are agents patterned after ___ (draw this).
Common features are N-1 alkylated 1,4 dihydro-3-carboxypyrid-4-one ring fused to another aromatic ring. Early quinolones where used to Tx ___ primarily. They achieve higher ___ concentrations compared to tissue/plasma. |
nalidixic acid
UTI urinary |
|
|
Newer quinolones are classified as ___, specifically ___ and are separate from the ___ specific agents.
|
fluoroquinolones, 6-fluoroquinolones, UTI
|
|
|
First generation quinolones are ___ absorbed orally, have ___ half-lives, and are ___ serum-protein bound, thus their use is restricted to:
Draw Oxolinic acid, class: Draw Cinoxacin, class: |
well, long, highly, protein free compartments like the urinary tract.
Oxolinic acid class: quinolone Cinoxacin class: dihydrocinnoline |
|
|
Second generation quinolones (fluoroquinolones), list them. Draw ciprofloxacin.
|
ciprofloxacin (drawn), norfloxacin, enoxacin, pefloxacin, lomefloxacin, ofloxacin, fleroxacin
|
|
|
Third generation quinolones include:
Draw levofloxacin |
levofloxacin (drawn), gatifloxacin, sparfloxacin
|
|
|
Fourth generation quinolones include:
|
trovafloxacin, moxifloxacin
|
|
|
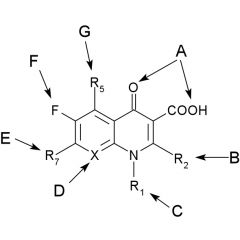
SAR quinolones
1. the 1,4-dihydro-4-oxo-3-pyridine carboxylic acid structure is essential for activity. The pyridine system must be annulated with an ___ ___. Isosteric replacements that retain activity, N for C at: C2, C5, C6, C8 give: |
aromatic ring.
C2: cinnolines (1,2-naphthyridine) C5: 1,5-naphthyridine C6: 1,6-naphthyridine C8: 1,8-naphthyridine |
|
|
SAR quinolones cont.
Substitution at C2 ___ activity. Sub at C5, C6, C7 and C8 gives ___ activity. Sub at C7 with piperazinyl or 3-aminopyrrolidinyl increases activity against ___ ___. |
abolishes/greatly decreases
favorable Pseudomonas aeruginosa |
|
|
SAR quinolones cont.
___ at C6 increased antibacterial activity. An alkyl sub at ___ is essential for activity. Aryl at ___ is also acceptable, see trovafloxacin. |
N1
N1 |
|
|
SAR cont.
Ring condensation on ciprofloxacin. Show product. |
ofloxacin
|
|
|
Antibacterial spectrum of early quinolones:
Gram ___ strains. resistant strains: |
negative
Gram-neg resistant strains: Pseudomonas aeruginosa, Haemophilus influenzae, Neisseria gonorrhoea |
|
|
Antibacterial spectrum of fluoroquinolones:
1. Sensitive gram-neg strains: 2. Sensitives gram-pos strains: |
1. Pseudomonas aeruginosa, Haemophilus influenzae, Neisseria gonorrhoea - all of which are resistant to quinolones
2. Staphylcoccus aureus and some streptococci |
|
|
Mech of action of quinolones.
1. Block DNA synthesis via inhibition of: 2. These enzymes normally catalyze transient DNA double-strand ___ staggered by ___ base pairs. 3. This decreases ___ of DNA and releases ___ ___. |
1. DNA gyrase (topoisomerase II) and topoisomerase IV
2. breaks, 4 3. supercoiling, torsional stress |
|
|
1. Inhibition of DNA gyrase and topoisomerase makes a cell's DNA ___ and leads to ___ ___.
2. DNA gyrase seems to be more important to gram-___ organisms while topoisomerase IV is more important to gram-___ organisms. 3. Human ___ is not very sensitive to the quinolones. |
1. inaccessible, cell death
2. negative, positive 3. topoisomerase II |
|
|
Topoisomerases:
Bacteria contain 4 important topoisomerases (I-IV) 1. Type I is made up of topoisomerase ___ and ___. 2. Type II is made up of topoisomerase ___ and ___. *we only focus on type II in this course |
1. I, III
2. II, IV |
|
|
1. Topoisomerase II (DNA gyrase) adds ___ ___ and removes ___ ___ making it easier for transcription and replication to occur.
2. Topoisomerase IV removes ___ ___, but can't add ___ ___. It functions to ___ daughter chromosomes (plasmids). |
1. negative supercoils, positive supercoils
2. positive supercoils, negative supercoils, decatenate |
|
|
1. Topoisomerase II and IV both consist of four subunits: 2 ___ and 2 ___.
2. Both have ___ and ___ activity. Thus they cause: |
1. alpha-subunits, beta-subunits
2. nuclease, ligase, double-strand breaks in DNA and then repair them. |
|
|
DNA gyrase assoc with DNA:
1. When bound to DNA gyrase, the DNA strands are cut in a ___ fashion, ___ base-pairs apart. 2. The cut ends are atached to ___ on each of the ___. 3. The beta subunits then hydrolyze ATP to provide energy for the conformation change that moves a section of DNA through the cut. 4. ___ interfere/block this process. |
1. staggered, 4
2. Tyr 122, alpha-subunits 4. fluoroquinolones |
|
|
Shen proposed MOA for quinolones.
1. 4 drug molecules self assemble only in the presence of the ___ complex. 2. They line up in aqueous environments with the 3,4,5,6 positions facing ___ towards the ___ ___ and the N1 substituents interacting with ___ in a ___ ___. |
1. DNA/gyrase
2. out, DNA bases, eachother, hydrophobic core |
|
|
Shen theory points:
1. Quinolones are more active if the N1 substituent is ___ and not large enough to interfere with the ___ of the molecules. 2. Substituents at positions 3,4,5,6 that increase ___ character will cause interference in the ___ ___ with DNA bases and lead to reduced activity. |
1. hydrophobic, stacking
2. hydrophobic, hydrogen bonding |
|
|
Resistance to quinolones is due to:
1. 2. 3. Resistance by plasmid mechanisms have not been observed. |
1. Reduced cellular uptake
2. Mutations in gyrA or gyrB which alter amino acid sequence in DNA gyrase may lessen sensitivity. 3. Actve efflux of quinolones |
|
|
QSAR studies.
In gram-negative bacteria there is an ___ relationship between uptake and logP, thus as lipophilicity increases, antibacterial activity ___. |
inverse, decreases.
|
|
|
QSAR studies.
In gram-positive bacteria there is a ___ correlation between uptake and logP, thus as lipophilicity increases, antibacterial activity ___. |
positive (direct), increases
|
|
|
Side effects of quinolones.
1. In ___ generation quinolones, a small % of patients experienced CNS effects; hallucinations, insomnia, visual disturbances, convulsions ect. 2. The ___ are generally better tolerated. |
1. first
2. fluoroquinolones |
|
|
Side effects of quinolones cont.
Compounds with ___ and ___ or a similar group at C7 have been associated with GABA receptor antagonism & exhibit a ___ ___. |
1-piperidinyl, 3-amino-1-pyrrolidinyl, proconvulsant activity
|
|
|
Side effects of quinolones cont.
Compounds with a halogen at C8 show greatest incidence of ___. Compounds with amino or methoxy at C5 or C8 show the lowest incidence. |
phototoxicity
|
|
|
Side effects of quinolones cont.
1. Quinolones have shown to cause erosion of ___ ___ ___ in young animals. 2. May cause severe ___ ___ and ___ ___ during first trimester of pregnancy. 3. Coadministration with ___ potentiates ___ actions. |
1. load bearing joints
2. metabolic acidosis, hemolytic anemia 3. theophylline, theophylline's |
|
Structure-side-effect relationships quinolone
|
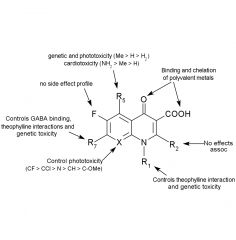
|
|
|
Physiochemical properties for fluoroquinolones
High than expected pKa values (5.6 - 6.4) the carboxyl group at C3 result from: |
Pseudoring H-bonding with C4 oxygen
|
|
|
At high doses and alkaline pH, norfloxacin and ciprofloxacin may cause ___ due to the increased concentration of ___.
|
crystalluria, zwitterion
|
|
|
Quinolones will ___ polyvalent metal ions, these are ___ water soluble and ___ bioavailable.
Coadministration with ___, ___, and ___ should be avoided. |
chelate, less, less, antacids, hematinics, dairy
|
|
|
Therapeutic applications
___, ___, and ___ are capable of developing resitance to quinolones. |
anaerobes, staphylcocci, pseudomonads
|
|
|
1. Cancer strikes more frequently with ___ ___.
2. ___ million Americans now living will eventually have cancer, it will strike ___ of ___ families. 3. In 2008, ___ Americans are expected to diagnosed with cancer, ___ of these live in Arkansas. These estimates exclude basal and squamous cell skin cancers and carcinoma in situ (noninvasive) of any site except urinary bladder. |
1. advancing age
2. 85, 3, 4 3. 1437180,14840 |
|
|
Cancer deaths in the US in 2008 are st to be ___.
|
565,650
|
|
|
1. Cancer is the second leading cause of death, exceeded only by ___. In the US, 1 of every ___ deaths is from cancer.
2. The 5-year survival rate for all cancers diagnosed btw 1996 & 2002 was ___. |
1. heart disease, 4
2. 66% |
|
|
1. Genetic theory of cancer: tumour development begins with changes in genetic information encoded in the cell either by ___, ___, or ___.
|
1. addition, subtraction, alteration
|
|
|
1. addition can be caused by:
2. alteration or subtraction can be caused by: |
1. cancer causing viruses
2. chemicals or radiation |
|
|
Nongenetic theory of cancer: 1. Alterations comparable with ___ ___ seen during development of organs and tissues of ___.
2. Such changes may give rise to cells with altered behaviour and apperance that can be passed from one ___ ___ to another. |
1. cellular differentiation, embryos
2. cellular generation |
|
|
1. Neoplasm:
2. Tumor: 3. Cancer: 4. Benign Tumors: 5. Malignant Tumors: |
1. Neoplasm: heritably altered, relatively autonomous growth of tissue.
2. Tumor: general term indicating any abnormal mass or growth of tissue. 3. Cancer: almost exclusively to indicate malignant neoplasm. 4. Benign Tumors: do not metastasize. 5. Malignant Tumors: metastasize. |
|
|
1. fibroma:
2. chondroma: 3. adenoma: 4. lymphoma: 5. hematoma: 6. granuloma: |
1. fibroma: benign tumor of fibrous tissue
2. chondroma: benign tumor of cartilage 3. adenoma: benign tumor of glandular tissue 4. lymphoma: (exception) - malignant tumor of lymphoid origin 5. hematoma: (Non-tumor exception) - localized mass of extravasated blood 6. granuloma: (Non-tumor exception) - nodular inflammatory lesion |
|
|
Embryonic layers.
1. ectoderm forms: 2. mesoderm forms: 3. endoderm forms: |
1. ectoderm forms: skin, appendages, and nerves
2. mesoderm forms: bone, muscle, cartilage ect 3. endoderm forms: gi tract and assoc organs |
|
|
1. Carcinomas come from which embryonic layer(s)?
2. Sarcomas come from which embryonic layer(s)? |
1. ectoderm, endoderm
2. mesoderm |
|
|
1. Tumor whose cells resemble all 3 germ layers - can be either malignant or benign
|
1. teratoma
|
|
|
Cancers of the blood:
|
leukemias, polycyhemia
|
|
|
Differences btw normal and cancer cells in vitro.
1. Normal cells must attach themselves to a surface and they grow until the form a ___. 2. Cancer cells lack ___ ___ and will grow and clump on top of each other. 3. Cancer cells are described as ___, whereas normal cells divide only through about ___ generations. |
1. monolayer.
2. mutual recognition. 3. immortal, 50 |
|
|
The cell cycle:
1. 2. 3. 4. 5. |
1. G1 - cell grows, protein synthesis occurs
2. S - DNA synthesis (replication) 3. G2 - Gap 2 - 2 copies of DNA separate (synth of RNA, protein, production of mitotic spindle) 4. M - mitosis 5. G0 - resting state - cell uses energy but doesn't grow, most cells normally in resting |
|
|
Two stage tumor development:
1. initiation: must be a ___ agent so that cell is likely to form a tumor upon exposure by a ___ agent. 2. promotion: This agent may be ___ and is an agent capable of altering ___ ___. |
1. carcinogenic, promoting
2. noncarcinogenic, gene expression |
|
|
Tumor dev:
Point mutations: 1. 2. Chromosomal mutations: 3. Genomic mutation: 4. |
Point mutations:
1. base pair sub 2. frameshift mut Chromosomal mutations: 3. alteration in gross structure of chromosome Genomic mutation: 4. change in number of chromosomes |
|
|
Tumor dev:
Addition of new genetic material is caused by ___. |
viruses
|
|
|
Change gene expression:
|
something turns genes on that are normally off or vice versa
|
|
|
C
A U T I O N |
Change in bladder/bowel habits
A sore that doesn't heal Unusual bleeding or discharge Thickening in breast or elsewhere Indigestion or difficulty swallowing Obvious change in wart/mole Nagging cough or hoarseness |
|
|
Five major classes of alkylating agents:
|
nitrogen mustards
ethyleneimines nitrosoureas alkyl sulfonates triazines |
|
|
MOA of nitrogen mustards:
a. Rate of cyclization is ___ if R = H or CH3. ___ if R = Ar ring or electron withdrawing group. b. Rate of nucleophile attack ___ than cycllization if R = H or CH3. Rate of nucleophile attack ___ than cyclization if R = Ar ring or electron withdrawing group. |
a. fast, slow
b. slower, faster |
|
|
Monofunctional alkylating agents - alkylation occurs at ___ of Guanine
|
N7
|
|
|
Normally guanine exists in DNA as the ___ tautomer, when alkylated, the ___ form may predominate leading to ___ base paring.
|
keto, enol, abnormal
|
|
|
Bifunctional alkylating agents form both ___ and ___ crosslinks in DNA. ___ is most likely responsible for the cytotoxicity.
|
intrastrand, interstrand, interstrand
|
|
|
1. ___ recognize alkylated or UV damaged bases as mistakes and nick the DNA at the damaged site.
2. ___ remove a section of damaged DNA 3. ___ ___ synthesize a new DNA segment. 4. ___ connect the 2 strands. |
1. endonucleases
2. exonucleases 3. repair polymerases 4. ligases |
|
|
Alkylating agents are cell phase ___, but they are most cytotoxic to proliferating cells in ___ or ___ ___ phase.
|
nonspecific, S, late G1
|
|
|
The nitrogen mustard Mechlorethamine is available in ___ dosage form. It readily cyclizes to the ___ ion. It is a potent ___. Avoid ___ contact and ___. ___ % disappears from the plasma in ___ minutes.
|
IV, immonium, vesicant, skin, extravasation, 90, 4
|
|
|
1. Cyclophosphamide is a ___.
2. Metabolically activated via ___ ___ ___ oxidation. 3. Not toxic ___ ___ unless ___ (serum supernatant fraction) is added. 4. Cyclophosphamide stimulates it's own ___. 5. One day Tx, T1/2 is 6.5 h, but by day 3-5 is decreased ___ %. |
1. prodrug.
2. Cyt P450 2B 3. in vitro, S9 4. metabolism 5. 30-40% |
|
|
Draw metabolic activation of cyclophosphamide.
1. metabolized by ___. Give toxic and non-toxic metabolites |
1. cyt P450 2B
|
|
|
Draw metabolic activation of Ifosfamide.
1. metabolized by ___. Give toxic and non-toxic |
1. cyt P450 3A
|
|
|
1. Ifosfamide and cyclophosphamide are urotoxic and cause ___ ___.
2. ___ is believed to be responsible for the toxicity. 3. Mesna (a nucleophile) is used to protect the bladder from metabolites such as Acrolein. Mesna is short for: |
1. hemorrhagic cystitis
2. Acrolein 3. Mercaptoethane sulfonic acid, Na salt |
|
|
Draw the Rxn of Acrolein with a nucleophile.
|
"michael rxn"
|
|
|
Draw mechanism of Mesna to detoxify Acrolein
|
*intermolecular disulfide bond in plasma - metab in kidney to active form
|
|
|
1. Estramustine Sodium Phosphate is used in advanced ___ cancer and metastatic ___ ___ ___.
2. The ___ metabolites most likely account for the in vivo effects - not the nitrogen mustard moeity. |
1. prostatic, renal cell carcinoma
2. estrogenic |

