![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
43 Cards in this Set
- Front
- Back
|
Are these organic or inorganic molecules? C6H1206, CH4 |
Organic |
|
|
Are these organic or inorganic molecules? NaCl, H20, CO2 |
Inorganic |
|
|
What elements make up an organic molecule? |
1. Carbon 2. Hydrogen 3. Oxygen |
|
|
If this molecule were composed only of hydrogen and carbon, how many hydrogens will there be? C--C--C |
8 |
|
|
Is this molecule hydrophilic or hydrophobic?
|
C6H12O6 |
|
|
True or False Two molecules with the formula C6H12O6 can be isomers. |
True |
|
|
True or false A molecule with the formula C5H10O5 can be an isomer of a moleucle with formula C6H12O6. |
False |
|
|
What element is the basis of all life on earth? |
Carbon |
|
|
Is carbon reactive or inert? Why? |
Carbon is reactive because it's most outer shell only has 4 atoms, it needs 8 to be inert. |
|
|
True or false Carbon cannot form single bonds with other carbons |
False, carbon CAN form bonds with other carbons. |
|
|
True or false Carbon atoms cannot make double bonds with some other atoms |
False. Carbon can make double bonds with other atoms. |
|
|
What are the 4 major types of organic molecules? |
1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids |
|
|
What two elements must be present in order for a molecule to be organic? |
Carbon and Hydrogen |
|
|
Are these an organic molecule? CO2 H20 Why? |
No, because they do not have both carbon and hydrogen combined. |
|
|
What does a carbon need to have in order to be functional? |
Carbon backbone |
|
|
What kind of carbon backbone is this? C-C-C-C-C-C |
Straight chain |
|
|
What kind of carbon backbone is this? C-C-C-C-C- | C |
Branched chain |
|
|
What kind of carbon backbone is this? -C-C / \ C C \ / -C-C |
Ring |
|
|
When you see H-N-H on a carbon chain, what does the H-N-H mean? |
Amino group |
|
|
When you see C-OH on a carbon chain, what does the C-OH mean? |
Carboxyl acid |
|
|
When you see O-H on a carbon chain, what does the O-H mean? |
Hydroxyl (alcohol) |
|
|
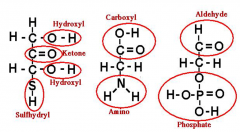
What are the 3 major functional groups in a carbon backbone?
|
Hydroxyl (OH), Amino (NH2), Carboxyl (CO2H) |
|
|
What function is this? H-C=O |
Aledhyde |
|
|
What function is this? C=0 |
Ketone |
|
|
What function is this S-H |
Sulfhydryl |
|
|
What function is this? H-O-P=O |
Phosphate |
|
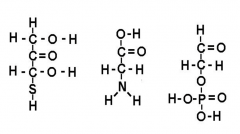
Circle and name functional groups |
|
|
|
What is the difference from hydrophillic vs. hydrophobic? |
Hydrophilic= Molecules interact with water Hydrophobic= Molecules do not interact with water |
|
|
Why is the ability of a molecule to interact with water a very important factor? |
Because most biological reactions take place in water |
|
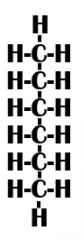
Is this function hydrophobic or hydrophilic? |
Hydrophobic |
|
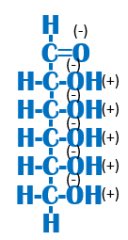
Is this function hydrophobic or hydrophilic? Why? |
Hydrophilic because it contains a lot of electronegative oxygens (-/+). Remember, a (-) and a (+) are ionic bonds which are soluble in water! |
|
|
What are isomers? |
Molecules that have the same molecular formula but different structural formula. |
|
|
What are two compounds (substrates/sugars) that are isomers? |
Glucose and Fructose. |
|
|
Given the molecular formula, C6,H12,O6 - could you tell if this is an isomer? |
Yes, they can be used to form either glucose or fructose. |
|
|
In order for a molecule to be an isomer it must have equal amounts of: |
It must have equal amounts of every compound. So if it has C, H, and O, it must have an equal amount of both. |
|
|
Is C6,H5,O7 an isomer? |
No |
|
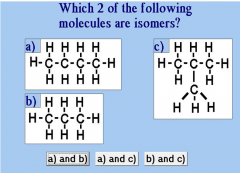
And why? |
A and C because they have equal amounts of Carbon (C) and Hydrogen (H) |
|
|
What are monomers?
|
A single subunit of a compound. Example, just one sugar. |
|
|
What is a dimer? |
Two sub-units joined together. So 2 monomers. |
|
|
What is a polymer? |
Many (at least more than 3) dimers joined together. |
|
|
What is dehydration synthesis? |
When you join two molecules (or compounds) together by removing water. A new product is created from reacting molecules. |
|
|
What does this molecular formula tell you? H2o / \ OH ----- H |
Dehydration synthesis is taking place. |
|
|
What is hydrolysis? |
When polymers and dimers are broken into monomers. It is essentially the oposite of dehydration synthesis. A molecule of water is split. |

