![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
70 Cards in this Set
- Front
- Back
|
Describe the defining characteristics of bacteria. Compare and contrast them to eukaryotic cells.
|
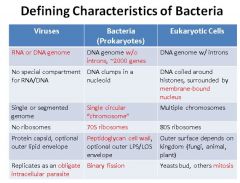
|
|
|
Outline the basic structure of bacteria including any special features.
|
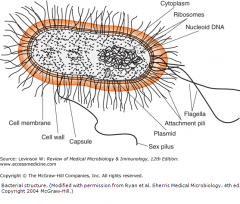
Cell membrane
Peptidoglycan cell wall (no sterols) Nucleoid; circular DNA chromosome No introns free floating ribosomes (70S) |
|
|
Describe the 3 major morphological types of bacteria. Give examples of each and describe their characteristic clumping patterns.
|
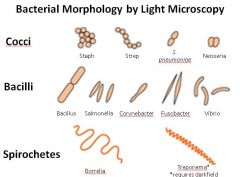
Note: Vibrio are a type of bacilli (curved rod or comma shaped);
Cocci: may arrange as grape-like clusters (Staph) or in chains (Strep), or in pairs (aka diplococci)--example: Neisseria form characteristic kidney bean shaped pairs. Bacilli (aka rods) |
|
|
Describe the steps of the gram staining procedure for bacteria. Define the test colors for a gram + vs. gram - bacterium.
|
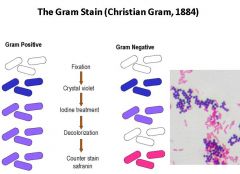
1. Fixation of bacteria to slide with heat
2. Stain with crystal violet (all bacteria turn purple) 3. Iodine tx (gram + will stay purple) 4. Decolorize using EtOH (leaches out purple from gram-) 5. Counterstain with safrinin (makes gram- visible again) Gram stain a fast, cheap way to narrow down differential and candidate pathogens |
|
|
Describe the differences in the external features of cell walls between Gram + and Gram - bacteria.
What is the role of peptidoglycan in bacteria? How is this exploited medically? |
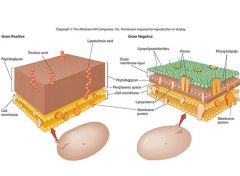
Peptidoglycan is unique to bacterial cells and it helps them retain their shape. It is exploited because it is a good drug target and well as target for innate immunity (i.e. lysozyme cleaves it). It is particularly vulnerable to the following drugs: cephalosporins, vancomycin, penicillins.
|
|
|
Describe the differences in the composition of the cell walls between Gram + and Gram - bacteria.
|
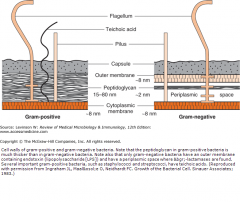
Gram +: no outer cell membrane, thick peptidoglycan layer, capsule, teichoic acid (can cause shock but rarer);
Gram -: Outer membrane, contains LPS/endotoxin (its LOS in Neisseria), thin peptidoglycan layer, has periplasmic space where beta lactamases are found. |
|
|
What is the third type of bacterial cell wall? Note: it does not stain gram + but is structurally different from other gram - bacteria.
|
Mycobacteria cell wall; stains with acid-fast stains. ex: mycobacterium tuberculosis
|
|
|
Describe the antigenic component of Gram + bacteria capable of causing shock.
|
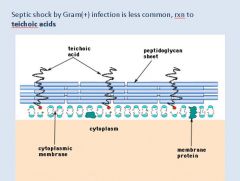
|
|
|
Describe the antigenic component of Gram - bacteria capable of causing shock? Which portion is use for laboratory ID of the bacterium?
|
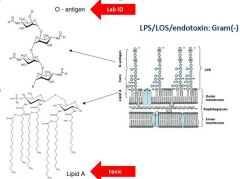
Lipid A--toxic/antigenic component
O-antigen used for lab ID |
|
|
Describe the two types of bacterial glycocalyces; How do they function as virulence or pathogenic factors for the bacteria?
|
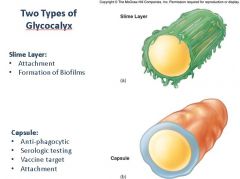
|
|
|
What are pili/fimbrae and what are they used for? Describe the pili of Gram + bacteria?
|
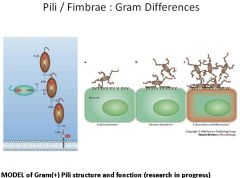
|
|
|
Describe the pili found in Gram - bacteria and their specialized functions.
|
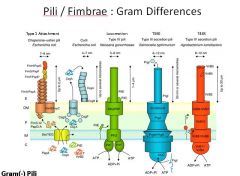
|
|
|
Define adjuvant.
|
Adjuvant is a compound that increases the immunogenicity of an antigen. The most common adjuvant used in vaccines is Alum, an aluminum salt.
|
|
|
Define passive immunization.
|
It is the use of a immune serum to give an immediate resistance to an infection.
|
|
|
Define active immunization.
|
It is the act of immunizing with antigens to induce an immune response to a pathogen.
|
|
|
What is a live-attenuated vaccine?
|
It is a vaccine that contains a weakened virus that can replicate to limited extent.
|
|
|
Bacteriostatic
|
Refers to antimicrobial agents that <i>inhibit growth</i> and/or reproduction of bacteria <b>but</b> <i>does not kill them</i>.
|
|
|
Bacteriocidal
|
Refers to an antimicrobial agent that <i>kills </i>or irreversibly damages bacteria.;
|
|
|
Narrow spectrum antiobiotic
|
Refers to antimicrobial agents that are active against select groups of bacteria.;<br />Examples: older penicillins, macrolides, vancomycin (only effective against G+ bacteria)
|
|
|
Broad spectrum antibiotic
|
Antibiotic with activity against a wide array or bacteria including G+ and G- bacteria.;<br />These drugs often used to treat patients "empirically" before causative bacteria is a ID'd and when treatment delay could be detrimental.;<br />Examples: carbapenems, extended spectrum cephalosporins, newer fluoroquinones.
|
|
|
Critical items
|
Devices that enter the bloodstream or sterile parts of body (i.e. surgical instruments, implants, invasive monitors). These have potential to transmit infectious agents and MUST BE STERILIZED.
|
|
|
Semi-critical items
|
Devices in contact with mucous membranes or intact skin; Free of spores; Includes respiratory and anethesia equipment. These have moderate potential for transmitting infectious agents and must receive HIGH LEVEL DISINFECTION.
|
|
|
Non-critical items
|
Devices that are in contact with intact skin but NOT mucous membranes; Includes bedpans, BP cuffs etc..These have limited potential for transmitting infectious agents and require LOW LEVEL DISINFECTION.
|
|
|
Antiseptic (antisepsis)
|
The process of reducing microorganisms on living tissue (i.e. skin). It does NOT kill spores. ;An antiseptic is a chemical used to kill microorganisms on surface of skin and mucous membranes.
|
|
|
Disinfection
|
The process of killing many but not all, pathogenic microorganism (i.e. spores may survive).
|
|
|
Sterilization
|
The process of killing or eliminating all microorganisms, including bacterial spores. This process is carried out in health-care facilities by physical or chemical means.;
|
|
|
List the 6 methods of sterilization.
|
1. Steam/Autoclave2. Dry Heat3. Gas Plasma (i.e. STERRAD system)4. Liquid Sterilization (i.e. STERIS system)5. Gas (Ethylene Oxide)6. Filtration<br />
|
|
|
For steam/autoclave sterilization:1. Technique2. Use/Advantages3. Disadvantages
|
1. 121 degrees/15psi for 15 mins (standard)2. Good for surgical instruments; rapidly microbicidal; penetrates medical packing, most device lumens3. Damages microsurgical or heat sensitive instruments.
|
|
|
For ethylene oxide gas sterilization:1. Technique2. Uses/Advantages3. Disadvantages
|
1. Gas covers device instruments;2. Ok for most medical materials; gets into device lumens3. Requires additional aeration time to remove ETO residue (toxic)
|
|
|
For gas plasma sterilization:1. Technique2. Uses/Advantages3. Disadvantages
|
1. ;Portable Sterrad chamber filled with gas plasma (hydrogen peroxide) in vacuum under UV or microwaves (creates free radicals). Sterilizes instruments in ~1 hour.2. Good for endoscopes or temperature sensitive materials. Nontoxic.3. Some types endoscopes with small, narrow lumens can't be processed; Cellulose, linens and liquids can't be processed.
|
|
|
For Liquid sterilization describe:1. Technique2. Uses/Advantages3. Disadvantages
|
1. ;35% liquid paracetic acid (PAA) used; instruments immersed and sterilant flows through removing microbes, salt, protein.2. Rapid cycle (30-45 minutes); low temperature3. Can only be used for immersible instruments; Must use when needed--no sterile storage available.
|
|
|
List the 5 mechanisms by which viruses efficiently and effectively use their genomes?
|
1. No wasted space in genome (i.e. no junk DNA or RNA)2. Overlapping reading frames3. Ribosomal frame shifting4. Alternative splicing of RNA5. Polyprotein cleavage
|
|
|
Why do viruses have a high mutation rate?
|
1. Simple polymerase makes many mistakes2. No proofreading or error correction3. Some viruses do not have a second strand (so nothing to compare/correct against)
|
|
|
What is the significance of viruses high mutation rate?
|
1. Mutations can produce new antigens that avoid current immunity (i.e. influenza strains; aka antigenic shift).2. Mutations can lead to drug resistance (i.e. protease inhibitor resistant mutants of HIV)3. Integration of viral genomes can cause disease<br />(In addition, virus mutations can allow live vaccines to be made and also help epidemiologists trace disease in a population)
|
|
|
List the 4 types of virus interactions that facilitate changes in the viral genome.
|
1. Complementation2. Phenotypic mixing3. Recombination4. Reassortment<br /><br />
|
|
|
Define: Complementation
|
When a gene function of one virus replaces a mutated gene of another. Allows defective viruses to replicate and express their genes.;
|
|
|
Define: Phenotypic mixing
|
Exchange of capsid proteins. For example if 2 polio virus serotypes infect the same cell, the progeny might have capsids that are a mixture of each serotype.;
|
|
|
Define: Pseudotype
|
The genetic material of one virus in the capsid or envelope of another.
|
|
|
Define: Recombination
|
The exchange of genes by crossing over at regions of homology. Produces a hybrid virus which reproduces. Example: western equine encephalitis virus.
|
|
|
Define: Reassortment
|
The rearrangement of parts of a segmented genome to form a new set of segments. Example: influenza virus (antigenic shift), reovirus.
|
|
|
What is viral interference? How is this accomplished?
|
Refers to the fact that infection with one virus tends to prevent infection by another. Due to: blocking of receptors, competition for resources, production of interferon or other antiviral agents (stimulates immunity)
|
|
|
In terms of mutation which types of genomes are most susceptible?
|
RNA genomes are most susceptible to mutation (inherently unstable)--they average 1 mutation per generation.;<br />DNA genomes are pretty stable averaging 1 mutation per several hundred or thousand generations.
|
|
|
What is a conditional lethal mutation?
|
A mutation that allows a virus to grow only under certain conditions (i.e. temperature sensitive mutants or host range mutants).
|
|
|
Autograft
|
A transfer of one's own tissue to another site on the body. There is no transplant rejection with this type of graft.
|
|
|
Syngeneic graft
|
A tranfer of tissue between genetically identical individuals (e.g. identical twins). This type of transplant usually "takes" --minimal (if any) rejection. Note: this type of transplant is often referred to as an isograft.;
|
|
|
Xenograft
|
A transfer of tissue between different species. This type of graft is always rejected by an <u>immunocompetent host.</u> Immunosuppressive drugs would be needed for it to take.
|
|
|
Allograft
|
A transfer of tissue between genetically different members of the same species (e.g. human to human). This type of transplant is rejected unless the recipient receives immunosuppressive drugs.
|
|
|
Graft-versus-host disease (GVHD)
|
A special type of rejection that occurs with bone marrow transplantation. ;Mature T cells in the <i>grafted </i>bone marrow attack the recipient's tissues.;
|
|
|
List the 4 blood types in the ABO system and indicated the antibodies that each makes.
|
<u>Blood Type</u>A: makes anti-B antibodiesB: makes anti-A antibodiesAB: makes no antibodies against A or BO: makes anti-A and anti-B antibodies
|
|
|
Hyperacute rejection
|
The most severe and immediate form of transplant rejection. Results from recipient's pre-existing antibodies reacting to the graft. Most commonly caused by ABO blood type mismatch. Alternatively, may result from mismatched HLA.;<br />Note: this type of rejection is sometime called "white graft" rejection due to the appearance of the rejected graft (i.e. becomes white due to loss of blood).
|
|
|
Acute rejection
|
A type of transplant rejection in which the <i>recipient's T cells</i> become<i> reactive against</i> the<i> graft. </i>This process may takes several days to weeks.<b> Strongest immune reaction results from graft tissue that expresses MHC class II. </b>May also result from antigen presentation by APC's via MHC class I.;<b><br /></b>Note: Acute rejection is the most common type of rejection. It is the focus of most immunosuppressive therapies.;
|
|
|
Chronic rejection
|
A type of transplant rejection that takes months or years to develop. It results from the recipient's indirect immune recognition of the graft via MHC molecules or minor transplantation antigens. ;It is usually associated with the presence of antibodies to HLA-class I antigens in the graft. This type of rejection acts on the vasculature of the graft.;
|
|
|
Describe the Panel Reactive Antibody (PRA) test. What is it used for?
|
The PRA is a quick test for HLA-antibodies. ;Serum from the transplant recipient is tested against a panel of donor leukocytes to see if they have antibodies to HLA. ;The test is presented as a percentage 0-100%. A low % is good, whereas a high % suggests potential difficulty in finding a suitable transplant donor (i.e. recipient has too many anti-HLA antibodies).
|
|
|
Describe cross-matching in the context of transplantation.
|
It is a test to compare compatibility between donor and recipient based on ABO blood type antigens. It is the most basic type of pre-transplantation matching that must be done.;
|
|
|
Describe the immunologic mechanism of hyperacute rejection.
|
<img src="paste-20315195310083.jpg" />
|
|
|
Describe the immunologic mechanism of acute rejection (direct pathway).
|
<img src="paste-20581483282435.jpg" />
|
|
|
Describe the immunologic mechanism of acute rejection (indirect pathway).
|
<img src="paste-20860656156675.jpg" />Indirect pathway: recipient APC process antigen from donor cells and initiate immune response/rejection.
|
|
|
Describe the immunologic mechanism for chronic rejection.
|
<img src="paste-21238613278723.jpg" />Note: chronic rejection acts mostly on vasculature of graft.
|
|
|
What is the most common type of transplantation?
|
Blood transfusions
|
|
|
List the 3 major classes of drugs used to immunosuppress transplant patients.
|
1. Corticosteroids2. Cytotoxic drugs3. Monoclonal antibodies
|
|
|
List the 2 most commonly used immunosuppressive corticosteroids and their mechanism of action.
|
Prednisone and Prednisolone; they work by interfering with transcription factor for T cell activation. This results in a "knocked down" T cell response.
|
|
|
Describe the major effects of corticosteroid therapy on the immune system.
|
<img src="paste-23519240912899.jpg" />
|
|
|
List the monoclonal antibodies used for immunosuppressive therapy.
|
1. Muromonab (Visilizumab): binds to CD3 molecule on T cells.2. Daclizumab or Basiliximab: target IL-2 receptor; inhibits activated T cells.
|
|
|
List the newest treatments for transplant immunosuppressive therapy. How do they work?
|
Belatacept (CTLA4-IG): recombinant DNA produced protein; combines CTLA4 extracellular portion with Fc region of human IgG1 antibody. MOA: blocks co-stimulation of T cells by covering up B7.;
|
|
|
Describe the mechanism of GVHD.
|
<img src="paste-24318104829955.jpg" />
|
|
|
Describe the tissues reactions in the 4 grades of GVHD.
|
<img src="paste-24567212933123.jpg" />
|
|
|
Identify and describe the 3 main classes of helminths?
|

|
|
|
Define the following types of parasitic hosts:
Intermediate Definitive Zoonotic |
Intermediate: A host in which the asexual cycle occurs or larva is present.
Definitive: a host in which the sexual cycle occurs or an adult is present. Zoonotic: a host that exists in the environment. |
|
|
List the 5 neglected parasitic diseases in the United States.
|

|
|
|
Identify the 5 classes of protozoa and their characteristic features.
|

|

