![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
|
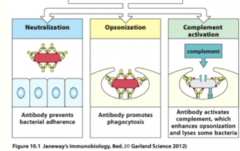
how do B-cell antibodies protect the body? (3)
|
|
|
|
B-cell activation
|
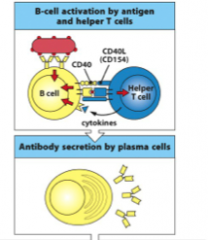
Antigen that binds to the B-cell antigen receptor both sends a signal into the B cell and is internalized and processed into peptides that activate effector helper T cells. Signals from the bound antigen and from the helper T cell (in the form of the co-stimulatory CD40 ligand (CD40L) and cytokines) induce the B cell to proliferate and differentiate into plasma cells secreting specific antibody
|
|
|
B-Cell Activation: Signal 1
|
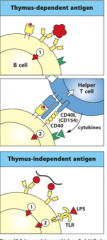
From the antigen (pathogen): cross-linking of B-cell receptors
2 Types: 1. Thymus-dependent Antigen (TD) -they require T-cell help; usually protein antigens (the majority of antigens) 2. Thymus-Independent Antigen (TI) -do NOT require T-cell help; usually has a repeating subunit structure: polysaccharides such as pneumococcal bacterial capsules **both types of antigens are required to cross-link BCRs to activate signal1!** - if the antigen is multivalent (multiple copies of one epitope) there is a greater chance the BCR binds to the same epitope and cross-links then the B cell phagocytoses the bacterium, degrades it, and presents it on MHC Class II |
|
|
B-Cell Activation: Signal 2- TD
|
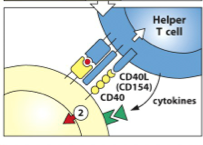
from helper T-cells: [CD40:CD40L] interaction
Thymus-Dependent Antigen (TD) -the [MHC II + Peptide] complex then interacts with the specific helper T cell (this helper T-cell has already been activated by a DC that presented the same [MHC II + peptide] sequence**) - once the helper T-cell binds with the [MHC II + peptide], it unregulated CD40 (on the B-cell) and CD40L (on the T-cell) and they interact to become Signal 2 |
|
|
How to Th cells affect B-cells?
|
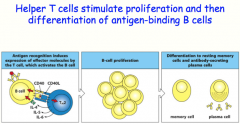
helper T-cells can secrete cytokines to induce somatic hypermutation and class switching
-the B-cell will differentiate into either a plasma cell OR a memory cell |
|
|
linked recognition
|
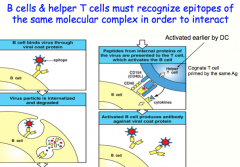
B cells and their cognate T-cells must have been exposed to the SAME Ag! Can bind to different epitopes but they recognize peptides/proteins from the same microbe
here: what the B-cell recognizes and attaches to is not what it actually presents to the T-cell (An epitope on a viral coat protein is recognized by the surface immunoglobulin on a B cell, and the virus is internalized and degraded. Peptides derived from viral proteins, including internal proteins, are returned to the B-cell surface bound to MHC class II molecules. These complexes are recognized by helper T cells, which help to activate the B cells to produce antibody against the coat protein. This process is known as linked recognition.) |
|
|
B-Cell Activation: Signal 2 -TI
|
signal 2 can be delivered multiple ways without involvement of T-cells
-repeating structure on the bacterium binds enough receptors to not only trigger signal 1, but also trigger signal 2 (these are TI-1 class of antigens) -DA or LPS from bacterium bind to Toll-like Receptors, delivering signal 2 |
|
|
how can we use linked-recognition to exploit the design of vaccines?
|
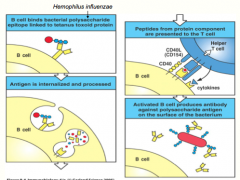
kids have a hard time responding to TI antigens, but a lot of bacterial pathogens like the lu are polysaccharide (TI) antigens
we can make a "Cognate Vaccine" -physically link the polysaccharide of H.Influenza to a protein (tetanus toxoid) -in this case, the BCR still recognizes the polysaccharide (so it initiates a response to it), but it also endocytoses the ENTIRE molecule, including the tetanus toxoid protein -the peptides from the toxoid are then presented on MHC II to initiate a T-dependent response, which included secretion of cytokines from the helper T-cell to undergo class switching *now the B-cell, specific for the polysaccharide are differentiated into plasma cells, capable of secreting IgG antibodies (due to the TD response induced by the toxoid); therefore being able to effectively opsonize and eliminate a future infection |
|
|
B-Cell activation in the spleen
|
Paracortex: T-cell seeks DC's to be activated by antigen- once activated, they express chemokines that direct them towards the follicle to encounter the B-cells
Follicle: B-cells are activated by antigen- once activate, they also express chemokines and migrate towards the boundary of the follicle and the T-cell area antigen-binding B cells meet T-cells a the border between the T-cell area and a B-cell follicle in secondary lymphoid some B-cells go to the medulla and differentiate into plasma cells to produce IgM as the first line of defense other B-cells activated by T-cells proliferate and form a germinal center in lymphoid tissue- this is where class switching a somatic hypermutation occurs, to compete for antigen among the activated B-cells |
|
|
B-cell activation in the lymph node
|
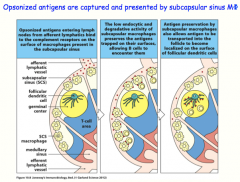
opsonized antigens are captured and presented to B-cells by subcapsular sinus macrophages
-preserve the antigen (instead of phagocytizing it!) to display for B-cells, or transfers to resident follicular dendritic cells, which also display antigen for the B-cell |
|
|
differences between a naive B-Cell and a Plasma Cell
|

plasmablast is a transition cell with the advantages of both
**plasma cells secrete Ig at a high rate, but no longer respond to Ag stimulation** |
|
|
overall cycle for a B-cell
|
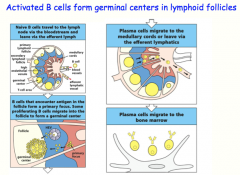
Naive B-cell -> [Lymph node]
-encounter antigen (delivered by SCS macrophage or follicular dendritic cell) -encounter T-cell -undergoes cell division --> some form "primary focus" (IgM)= early immune response --> rest will go into the follicle and form the germinal center -somatic hypermutation -class switching (with help from T-cell) Terminally differentiate into plasma cells -> back to Bone Marrow -produce antibodies for secretion |
|
|
somatic hypermutation
|
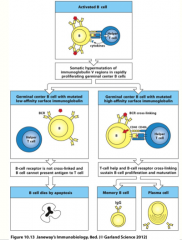
activated B cells in germinal center undergo rounds of mutation and selection for higher affinity mutants, resulting in high-affinity antibody-secreting plasma cells and high-affinity memory B cells
can only occur with helper T-cell activation! |
|
|
class switching
|
different cytokines induce switching to different isotopes of immunoglobuulins
not only to cytokines have the ability to STIMULATE switching to certain types of immunoglobulins, but they also can INHIBIT others! the different classes of immunoglobuliins attach to different cell types with differing affinities. Remember that since only the CONSTANT REGION is changed, this would be logical since the different cells bind to the Fc region |
|
|
what isotype does IL-4 induce?
|
IgG1, IgE
|
|
|
what isotype does IL-5 induce?
|
IgA
|
|
|
what isotype does IFN-gamma induce?
|
IgG3, IgG2a
|
|
|
what isotype does TGF-beta induce?
|
IgG2b, IgA
|
|
|
TI-1 class of antigens
|
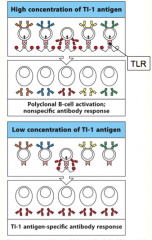
are capable of activating all B cells by cross-linking Toll-Like Receptors on the B-cell surface, and causing them to differentiate and proliferate
at high concentrations they activate B cells indiscriminately, causing polyclonal activation (all B-cells are activated!) at lower concentrations they function like normal antigens, and only activate those B cells whose receptors have an affinity for the antigen (only B-cells specific for the TI-1 antigen respond) |
|
|
TI-2 class of antigens
|
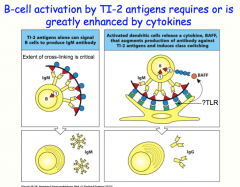
TI-2 antigens tend to have a lot of repeating subunits
they are capable of ligating many B cell receptors at once like TI-1 antigens, TI-2 antigens induce B-cell to secrete IgM antibodies without other stimuli, TI-2 cannot induce class switching when paired with cytokines, TI- 2 antigens can provide the necessary signal 2 to lead to class switching and the production of IgG |
|
|
NK Cells and Antibody Dependent Cellular Cytotoxicity (ADCC)
|
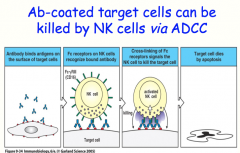
NK cells are the principle immune response in the absence of MHC molecules, like in viral infection or malignancy
they can kill cells via Antibody Dependent Cellular Cytotoxicity (ADCC) |

