![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
216 Cards in this Set
- Front
- Back
|
Cytomegalovirus
|
cyto-, "cell", and -megalo-, "large") is a viral genus of the viral family known as Herpesviridae or herpesviruses. It is typically abbreviated as CMV.
|
|
|
How CMV infects
|
Infected through direct contact, exposures to secretions, blood transfusions
|
|
|
How CMS infects fetus
|
Fetal infection via placenta
|
|
|
Early exposure increases fetal risk
|
30-40% risk of transmission to fetus
20-25% risk post natal |
|
|
CMV causes
|
-The Most Common infectious cause of mental retardation, deafness, visual impairment
-Detectable abnormalities in fetus associated with poor neurodevelopmental outcome -Mortality 30-60% within 2 years |
|
|
Are infants symptomatic from CMV?
|
90% of congenitally infected infants are asymptomatic at birth
Neurologic issues found in 30% of these infants within 1st year of life |
|
|
Assumed fetal infection in documented maternal infection IF...
|
Progressive IUGR
Microcephaly Hepatomegaly/splenomegaly Cerebral & visceral calcifications Hydrops |
|
|
CMV Ultrasound findings
Brain |
Ventriculomegaly
Periventricular echogenicity Calcifications – often non shadowing Microcephaly Cerebellar/CM abnormalities Periventricular cystic changes |
|
|
Diagnosis of fetal infection
|
via amnio
Performed at least 7 weeks after the assumed infection |
|
|
Evaluate Baby for
|
growth (IUGR), anemia (MCA), fetal hydrops
|
|
|
Parvovirus
|
20-30% of women infected transmit to fetus
Risk of fetal death highest (20%) when transmitted <20 weeks |
|
|
Parvovirus causes
|
Normal developmental outcome in most children who survive infection
Neurodevelopmental delays reported in severe infection |
|
|
Ultrasound for Parvovirus
|
hydrops, anemia
Transfusions when anemic (shown via MCA) |
|
|
Parvovirus Ultrasound
|
Ascites
-Can progress to hydrops cardiac failure from severe anemia -Placentomegaly -Polyhydramnios -Echogenic bowel |
|
|
Varicella
|
Chicken Pox virus
|
|
|
Maternal infection <20 weeks 6% fetal transmission
|
1/3 of infected fetuses have clinical manifestations, usually cutaneous
1-2% of fetuses have severe clinical findings Children can be neurologically asymptomatic Neurologic impairment depends on location and extend of lesions |
|
|
Varicella Ultrasound
|
-Intrahepatic and intracranial calcifications
-Polyhydramnios from neurologic impairment that hinders swallowing -Limb hypoplasia, contractures |
|
|
Varicella Ultrasound
|
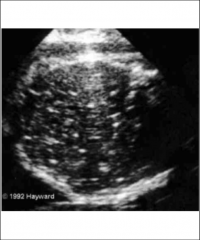
|
|
|
Amniotic Band Syndrome
|
Entrapment of fetal parts by disrupted amnion
|
|
|
Current theory is rupture of amnion
|
Chorionic side of membrane is sticky > entrapment of fetal parts > vascular constriction >deformity/amputation
Occurs between 6-18 weeks |
|
|
Treatment
|
possible in utero lysis of bands
|
|
|
ABS imaging
|
Asymmetric disruption of defects is hallmark of syndrome
Craniofacial deformities often severe – often asymmetric Abdominal wall defects Edema of distal extremities secondary to constriction May progress to limb amputations Amniotic band in contact with deformity |
|
|
ABS ultrasound
|
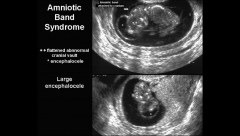
http://www.sonoworld.com/TheFetus/page.aspx?id=1658
|
|
|
Beckwith-Wiedemann Syndrome
|
Multigenetic disorder
|
|
|
Beckwith-Wiedemann Syndrome Classic Triad
|
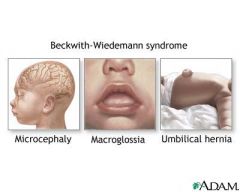
Macrosomia
Omphalocele (usually small) Macroglossia |
|
|
Beckwith-Wiedemann Syndrome Other Findings
|
Nephromegaly – but normal echogenicity
Hepatomegaly Hemihyperplasia Ear groove Polyhydramnios |
|
|
BWS Risks
|
Increased maternal risk Preeclampsia
Increased risk for PTD Increased risk of Wilms Tumor Hypoglycemia (infant) http://www.sonoworld.com/TheFetus/page.aspx?id=2527 |
|
|
BWS ultrasound
|
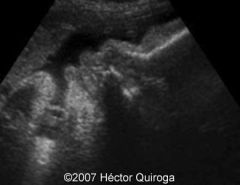
|
|
|
Meckel-Gruber Syndrome
|
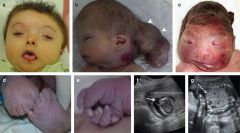
Autosomal recessive
|
|
|
Meckel-Gruber Syndrome classic triad
|
Classic triad:
Renal Cystic Dysplasia Cephalocele Polydactyly Should have 2/3 classic features |
|
|
Ability to properly evaluate abnormalities hindered by severe
|
oligohydramnios to anyhydramnios
|
|
|
Meckel Gruber Syndrome Genitourinary Tract
|
Renal cystic Dysplasia most consistent finding
Grossly enlarged, echogenic 10-20x normal size +/- large (visual) cysts Large AC Bladder/stomach small/absent Usually anhydramnios |
|
|
MGS CNS
|
Occipital Encephalocele – 60-80%
Dandy Walker abnormalities Microcephaly Agenesis CC Ventriculomegaly Holo |
|
|
MGB Extremities
|
Postaxial Polydactyly
The most difficult to see secondary to anhydramnios Clubbed feet Short limbs Bowing long bones |
|
|
MGS Facial Malformations
|
Cleft lip/palate
Micrognathia Micropthalmia Micrognathia Ear malformations Sloping forehead |
|
|
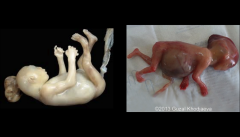
Sirenomelia AKA mermaid syndrome
|
Rare, usually lethal malformation characterized by varying degrees of lower extremity fusion and associated skeletal, GI, and genitourinary abnormalities
|
|
|
possible causes of Sirenomelia
|
Possible a vascular accident
Blood diverted away from lower extremities in early embryology Possibly caused by abnormality in blastogenesis Very early defect due to disruption of caudal mesoderm in 3rd week of gestation |
|
|
Sirenomelia imaging
|
Single or fused lower extremities
Absence of normally tapered lumbosacral spine Mid trimester anhydramnios due to bilateral renal agenesis or other renal abnormalities 2VC |
|
|
Sirenomelia seen when?
|
Findings can be seen in 1st trimester
|
|
|
Ultrasound image of sirenomeila
|
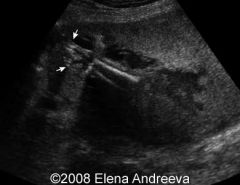
|
|
|
Tuberous Sclerosis
|
Genetic tumor disorder
|
|
|
Tuberous Sclerosis Genetics
|
Autosomal dominant
>50% new mutations Variable expressivity |
|
|
Tuberous Sclerosis causes
|
Rhabdomyomas
Brain lesions Kidney lesions |
|
|
Cardiac Rhabdomyomas
|
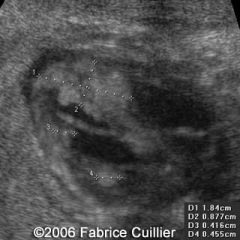
Multiple rhabdo’s = 100% TS
Single rhabdo = 50% TS Tumor involves ventricles or IVS As early as 22 weeks Arrhythmias |
|
|
Outflow or inflow tract obstruction
|
> congerstive heart failure > hydrops > death
|
|
|
Renal lesions
|
Cysts
Angiomyolipomas Appreciated after birth |
|
|
Skeletal Dysplasia
|
Defined as abnormal growth & density
|
|
|
Dwarfism occurs secondary to?
|
Skeletal Dysplasia
|
|
|
Lethal forms of SD are ?
|
severe in their presentation
|
|
|
Nonlethal SD are less ?
|
severe in appearance
|
|
|
Assess limb shortening
|
measure all long bones (R/U/T/F/H/Fm)
|
|
|
Dysplasia if
|
2 standard deviations below mean
|
|
|
Assess bone contoure
|
thickness, abnormal bowing, fractures, ribbon-like appearance
|
|
|
Degree of Ossification?
|
: hypomineralization
|
|
|
Thoracic Circumference & Shape looks like?
|
Narrow chest, Bell shaped
|
|
|
SD Hand & Foot Anomalies
|
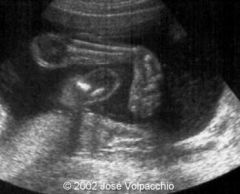
Talipses, polydactyly
|
|
|
SD Face & Profile
|
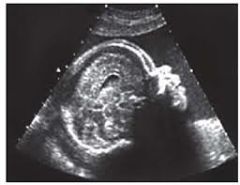
Clefts, frontal bossing, micrognathia, hypertelorism
|
|
|
Other SD anomalies
|
Hydrocephalus
Heart defects Hydrops? |
|
|
Rhizomelia
|
Shortening of proximal bone (humerus, femur)
|
|
|
Mesomelia
|
Shortening of middle segments (R/U/T/F)
|
|
|
Micromelia
|
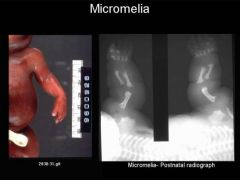
Shortening of entire extremity
|
|
|
Thanatophoric Dysplasia
|
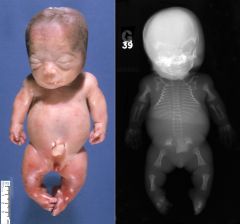
severe skeletal disorder characterized by a disproportionately small ribcage, extremely short limbs and folds of extra skin on the arms and legs.
|
|
|
most common form of lethal SD
|
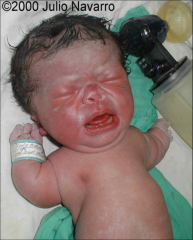
Thanatophoric Dysplasia
|
|
|
Thanatophoric Dysplasia Type 1:
|
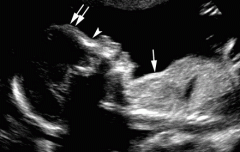
Curved, short femurs; flat vertebral bodies
|
|
|
Thanatophoric Dysplasia Type 2:
|
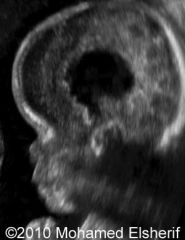
Straight, short femurs; flat vertebral bodies; cloverleaf skull
|
|
|
Thanatophoric Dysplasia US Findings
|
Severe micromelia
Cloverleaf Skull Narrow Thorax w/ shortened ribs Protuberant Abdomen Frontal Bossing Hypertelorism Flat Vertebral Bodies Polyhydramnios Hydrocephalus Hydrops |
|
|
Narrow Thorax
|
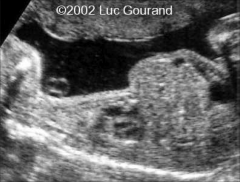
|
|
|
Shortened Ribs
|
|
|
|
Achondroplasia
|
Results from decreased endochondrial bone formation
Usually spontaneous mutation; can be autosomal recessive |
|
|
The most common nonlethal SD
|
Achondroplasia
|
|
|
Advanced paternal age increases risk of?
|
Achondroplasia
|
|
|
Achondroplasia Prognosis
|
depends on type
|
|
|
Heterozygous Achondroplasia:
|
good survival rate, normal intelligence
May require orthopedic or neurologic surgical intervention |
|
|
Homozygous Achondroplasia
|
Lethal
US findings more severe, including narrow thorax |
|
|
US Findings: *MAY NOT BE EVIDENT UNTIL AFTER ?
|
22 WEEKS!!!*
|
|
|
Achondroplasia US Findings
|
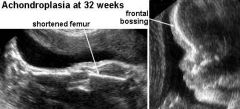
Rhizomelia
Macrocephaly Trident Hands (short proximal/middle fingers) Depressed nasal bridge Frontal Bossing Mild Ventriculomegaly |
|
|
Achondrogenesis
|
is a number of disorders that are the most severe form of congenital chondrodysplasia (malformation of bones and cartilage). These conditions are characterized by a small body, short limbs, and other skeletal abnormalities.
|
|
|
Rare, lethal SD
|
Achondrogenesis
|
|
|
Achondrogenesis cause
|
Caused by cartilage abnormalities that result in abnormal bone formation and hypomineralization
|
|
|
Achondrogenesis type 1
|
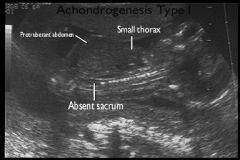
: More severe, autosomal recessive
|
|
|
Achondrogenesis type 2
|
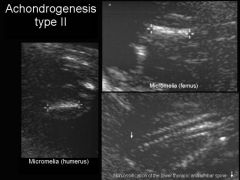
Less severe, spontaneous mutation
|
|
|
Achondrogenesis Ultrasound
|
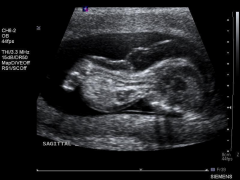
US Findings:
Severe micromelia Decreased or absent ossification of spine Macrocephaly Short trunk Short thorax & Short Ribs Micrognathia Polyhydramnios Hydrops |
|
|
Achondrogenesis Ultrasound
|
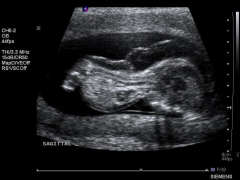
|
|
|
Achondrogenesis Ultrasound Legs
|
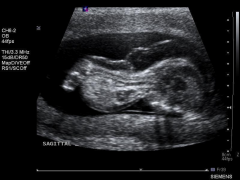
|
|
|
Achondrogenesis Ultrasound arm
|
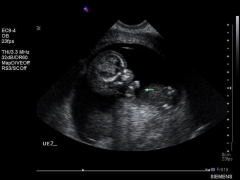
|
|
|
Osteogenesis Imperfecta
|
Rare disorder of collagen production leading to brittle bones
|
|
|
Osteogenesis Imperfecta Manifests in ?
|
teeth, skin, ligaments, blue sclera
|
|
|
Osteogenesis Imperfecta has how many types?
|
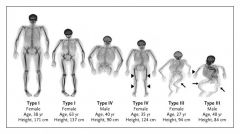
4 types
|
|
|
Osteogenesis Imperfecta Type 1:
|
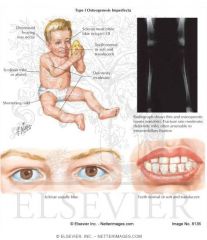
Autosomal Dominant, mild presentation. Not likely diagnosed prenatally
|
|
|
Osteogenesis Imperfecta Type 2
|
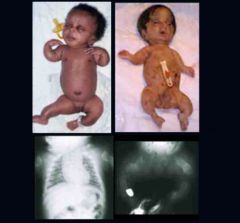
Most severe; lethal. May be dominant, recessive, spontaneous mutation
|
|
|
Osteogenesis Imperfecta type 3
|
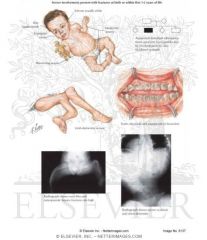
Severe. May be dominant or recessive
|
|
|
Osteogenesis Imperfecta type 4
|
Autosomal Dominant, also mild presentation. Similar to type 1; not likely diagnosed prentally
|
|
|
Prognosis of Osteogenesis Imperfecta
|
Type 1 & 4: multiple fractures, short stature
Type 1 children may also suffer from kyphoscoliosis & deafness Type 2: Infants die shortly after birth secondary to respiratory complications Type 3: produces significant handicaps with progressive deformities of long bones and spine |
|
|
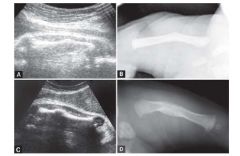
Osteogenesis Imperfecta Type 2 US Findings
|
Generalized hypomineralization, especially calvarium (compressible)
Multiple fractures: long bones, ribs, spine Narrow thorax Micromelia Polyhydramnios |
|
|
Osteogenesis Imperfecta Type 3 US Findings
|
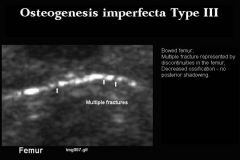
Similar to type 2 but less severe
|
|
|
Short-Rib Polydactyly Syndrome
|
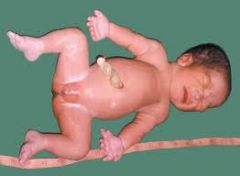
Lethal SD
Short ribs, short limbs, polydactyly Autosomal Recessive |
|
|
US Findings
Short-Rib Polydactyly Syndrome |
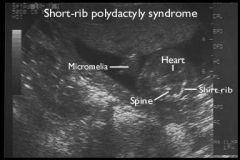
Narrow thorax, short ribs
Polydactyly Micromelia Midline Facial Cleft |
|
|
Other Short-Rib Polydactyly Syndrome findings can include:
|
CNS anomalies
Cardiovascular system Genitourinary tract Polyhydramnios |
|
|
Postural Anomalies
|
The abnormal movements can also lead to abnormal contractures & postural deformities
|
|
|
Normal development requires
|
normal movement
|
|
|
Decreased fetal movement caused by:
|
Oligohydramnios
Multiple gestations Congenital uterine anomalies Fetal Nerves Connective Tissue Musculature |
|
|
Arthrogryposis Multiplex Congenita
|
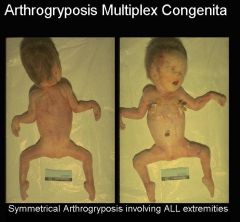
Severe contractures of extremities b/c of abnormal innervation, muscles, & connective tissue
|
|
|
Arthrogryposis Multiplex Congenita caused by?
|
May be sporadic or inherited
|
|
|
Arthrogryposis Multiplex Congenita Prognosis
|
Varies
|
|
|
US Findings Arthrogryposis Multiplex Congenita
|
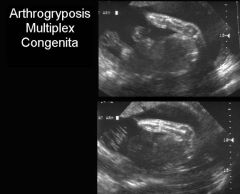
Rigid Extremities
Flexed Arms Hyperextension of knees Clinched Hands Talipses Poly/oligo +/- CNS, renal, facial Anomalies |
|
|
US Findings Arthrogryposis Multiplex Congenita
|
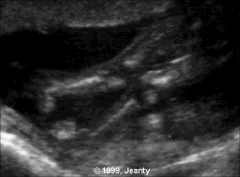
|
|
|
Lethal Multiple Pterygium Syndrome Characterized by ?
|
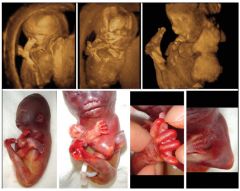
webbing Across joints & multiple contractures
|
|
|
Lethal Multiple Pterygium Syndrome is autosomal ________?
|
Autosomal Recessive
|
|
|
Lethal Multiple Pterygium Syndrome US Findings
|
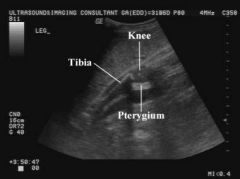
Limb Contractures
Webbing Across Joints Cystic Hygroma |
|
|
Misc Limb Abnormalities Hand Anomalies
|
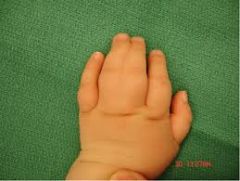
Syndactyly: fused fingers
|
|
|
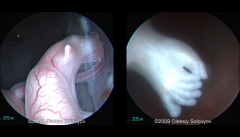
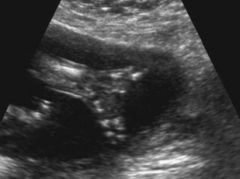
Ectrodactyly
|
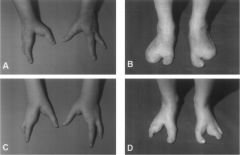
AKA Split Hand orLobster Claw Deformity
|
|
|
Ectrodactyly ultrasound
|
|
|
|
Missing digits
|
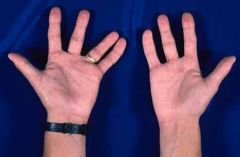
Hypodactyly
|
|
|
Polydactyly:
|
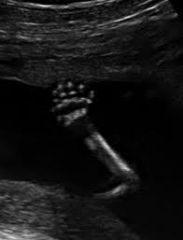
too many digits
|
|
|
Clinched Hands
|
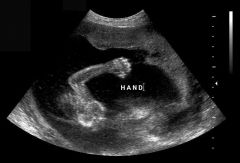
|
|
|
Talipes
|
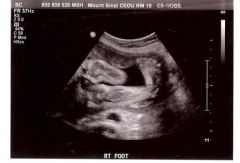
club foot
|
|
|
Talipes Findings and causes
|
Half are unilateral
Male predominance Usually idiopathic & isolated Can be associated with chromosomal Abnormalities, NTD’s, syndromes, musculoskeletal disorders Oligohydramnios, multiple gestations |
|
|
Rocker Bottom Foot
|
Prominent Heel and a convex sole
|
|
|
Rocker Bottom Foot Associated with ?
|
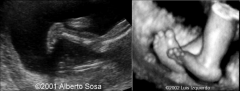
multiple syndromes, chromosomal abnormalities (T18!!)
|
|
|
Environmental teratogens increase risk by?
|
7%
|
|
|
Most SENSITIVE period for cardiac development?
|
3 ½ - 6 ½ weeks
|
|
|
Cardiovascular system 1st organ system to reach _______ _______.
|
functional state
|
|
|
At end of which week blood is circulating
|
3rd
|
|
|
Which week heart starts to beat?
|
5th
|
|
|
Fetal circulation
|
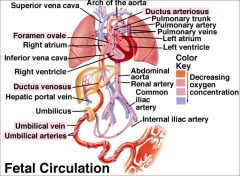
|
|
|
Foramen Ovale:
|
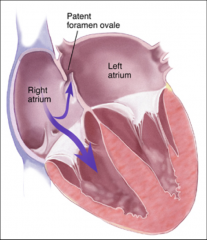
Opening between two atrias, blood flows from right to left
|
|
|
Ductus Arteriosus:
|
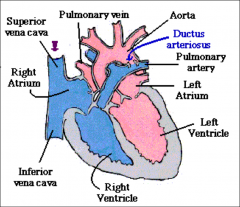
Communication b/t pulmonary artery & descending aorta
Closes after birth |
|
|
Ductus Arteriosus ultrasound
|
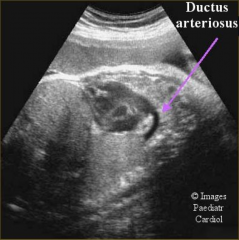
|
|
|
DA Ultrasound
|
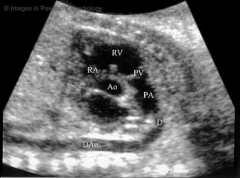
|
|
|
Fetal Risk Factors:
|
IUGR
Cardiac Arrhythmias Abnormal Amnio (trisomy) Abnormal Heart Rate Other anomalies Renal anomalies GI Anomalies When more than 1 abnormality present, the risk for cardiac defects increases further |
|
|
Maternal Risk Factors
|
Fam Hx cardiac defect
DM (VSD, Transposition GA’s, ToF) Lupus Teratogen (lithium, Alcohol) |
|
|
Familial Risk Factors:
|
Genetic Syndromes, heart defect in previous child
Parent with congenital heart defect |
|
|
Normal Heart Images
|
Cardiac Axis
Situs 4C View LVOT RVOT Aortic Arch M-Mode |
|
|
cardiac normal axis
|
45 degree axis from spine
|
|
|
Heart normal size
|
Cardiac circumference is ½ chest circumference
Small chest or large heart? |
|
|
Four-Chamber view
|
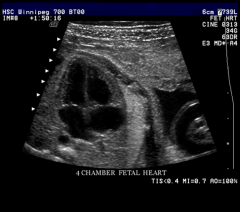
Apex pointed to left
Right side slightly larger than left Foramen Ovale flap visualized bowing into left atrium Moderator band identified in right ventricle Mitral and tricuspid valves assessed Tricuspid valve sits slightly inferior to mitral valve Pulmonary veins seen entering Left Atrium Right lower vein not imaged SVC & IVC enters right atrium Interventricular Septum |
|
|
4 chamber view
|
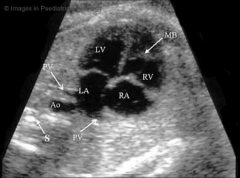
|
|
|
normal 4chamber view clip
|
http://www.youtube.com/watch?v=8lnuJvlDSE0
|
|
|
Examples of normal AND abnormal
|
http://www.centrus.com.br/DiplomaFMF/SeriesFMF/doppler/capitulos-html/chapter_12.htm
|
|
|
Things to consider with out flow track
|
Size/width of OFT’s
Position |
|
|
LVOT Standard view:
|
Long Axis
|
|
|
RVOT Standard View
|
Short axis
Sometimes Long axis |
|
|
LVOT US
|
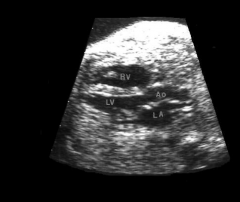
http://www.youtube.com/watch?v=hfJOZ7FJMqU
|
|
|
RVOT
|
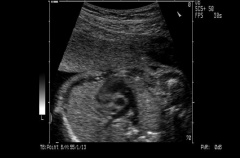
http://www.medison.ru/uzi/eho393.htm
Right Ventricular Outflow Tract |
|
|
OFT’s Crossing
|
http://www.youtube.com/watch?v=AfDTNciZl9g
|
|
|
AO arch
|
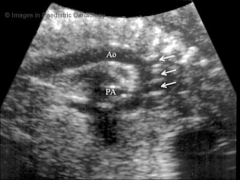
|
|
|
Levocaria:
|
normal position
|
|
|
Levoposition
|
Heart displaced further left than normal
|
|
|
Dextrocardia
|
Heart in right chest, apex points right
|
|
|
Dextroposition:
|
Heart is in right chest, apex points medially or left
|
|
|
Mesocardia
|
Apex points midline
|
|
|
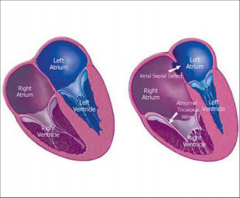
Ventricular Septal Defect - VSD
|
Defect (hole) in the septum of the heart
|
|
|
20% of all congenital heart disease
|
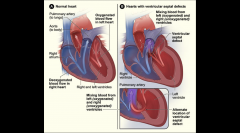
Ventricular Septal Defect - VSD
|
|
|
Associated cardiac abnormalities 50%
|
Ventricular Septal Defect - VSD
|
|
|
Ventricular Septal Defect - VSD Membranous
|
More common – 75%
high up in ventricle (near crux) in outflow tract of left ventricle, immediately below aortic valve |
|
|
VSD Muscular:
|
10-15%
Defect in muscular portion of of septum, anywhere from apex to to base Large, small, single, many |
|
|
% of VSD’s close within 2 yrs of life
|
40
|
|
|
% close by 5 yrs
|
60
|
|
|
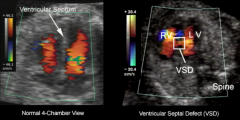
VSD US
|
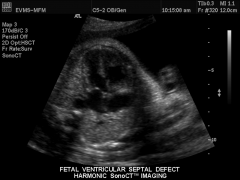
|
|
|
Atrioventricular Septal Defect - AVSD
|
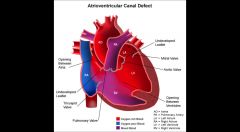
AKA endocardial cushion defect
deficiency of the atrioventricular septum of the heart. It is caused by an abnormal or inadequate fusion of the superior and inferior endocardial cushions with the mid portion of the atrial septum and the muscular portion of the ventricular septum. |
|
|
Atrioventricular Septal Defect - with T21
|
40% of cases
|
|
|
ASVD prognosis with surgery
|
Excellent
|
|
|
AVSD Imaging Best diagnostic clue
|
= missing crux of heart
|
|
|
AVSD imaging Color doppler:
|
Confirm defects
Regurgitation http://www.youtube.com/watch?v=921fShockXI |
|
|
US normal vs. AVSD
|
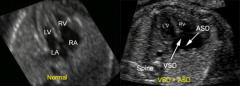
|
|
|
AVSD US
|
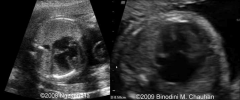
|
|
|
Ebstein Anomaly
|
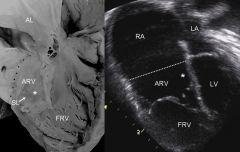
Apical displacement of septal and posterior tricuspid valve leaflets
Valve leaflet lowered into right ventricle Results in atrialization of RV - congenital heart defect in which the septal leaflet of the tricuspid valve is displaced towards the apex of the right ventricle of the heart. |
|
|
In utero mortality rate of Ebstein Anomaly
|
45%
|
|
|
Absence of antegrade flow across pulmonary valve is
|
lethal
|
|
|
Ebstein Anomaly Best Imaging Clue
|
Right Atrial Enlargement***
Apical Displacement of tricuspid valve*** sits lower in heart than normal Normal = 1-2mm lower than mitral valve |
|
|
Ebstein Anomaly - Imaging
|
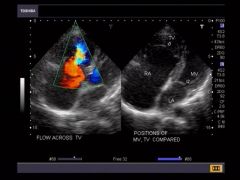
Cardiomegaly
Right atria large while right ventricle small from atrialization – only a small amount of functional right ventricle left Pulmonary artery often from lack of flow Color Doppler to eval regurg through tricuspid valve |
|
|
Ebstein Anomaly - Imaging
|
http://www.sonoworld.com/TheFetus/page.aspx?id=3002
|
|
|
Hypoplastic Left Heart
|
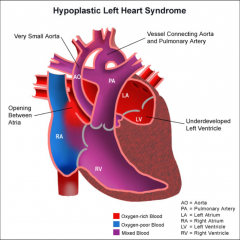
|
|
|
Hypoplastic Left Heart
|
|
|
|
Hypoplastic Left Heart
|
Underdeveloped left side of the heart (LV + LA)
Mitral stenosis/atresia Aortic stenosis/atresia Hypoplastic ascending Ao and coarctation |
|
|
Hypoplastic Left Heart Lethal if
|
-untreated
20% IUFD Improving surgical techniques = 80 to near-100% mortality Long term survival unknown |
|
|
Hypoplastic Left Heart Aneuploidy:
|
13% turners
T13/18 |
|
|
The amount of hypoplasia depends
|
on when the left sided atresia developed
|
|
|
Hypoplastic Left Heart, Right vent supplies both
|
pulmonic and systemic blood flow
|
|
|
Hypoplastic Left Heart Pulmonary veins return blood to
|
right atrium
|
|
|
Hypoplastic Left Heart:nOverload of right vent may lead to
|
CHF >Pericardial effusions > Hydrops
|
|
|
Hypoplastic Left Heart - Imaging
|
Abnormal 4-chamber view
Most cases detected at anatomy scan |
|
|
Hypoplastic Left Heart - Imaging Left ventricle:
|
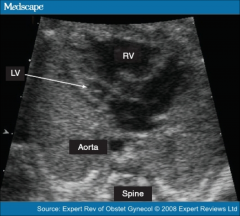
Small or nonexistent
Hypocontractile and hypertrophic |
|
|
Hypoplastic Left Heart Right ventricle
|
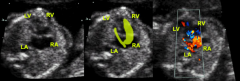
Dilated and often wraps under left ventricle apex
Hypertrophied with good function |
|
|
Hypoplastic Left Heart Atria
|
Interatrial septum bowed left to right
Only outlet for flow from left atrium Left atrium is hypertrophic, right atrium dilated |
|
|
Hypoplastic Left Heart Pulmonary artery
|
dilated
|
|
|
Hypoplastic Left Heart Ductuc arteriosus (DA)
|
dilated
|
|
|
Hypoplastic Left Heart Ascending aorta
|
small
Associated with coarctation |
|
|
Hypoplastic Left Heart case
|
Case Report
http://www.sonoworld.com/TheFetus/page.aspx?id=2683 http://www.sonoworld.com/TheFetus/page.aspx?id=2525 |
|
|
Congenital heart disease with 4 components
|
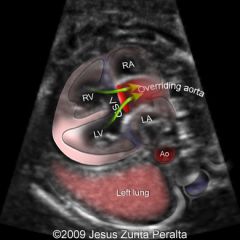
Tetralogy of Fallot
|
|
|
Tetralogy of Fallot
|
RVOT obstruction/stenosis
VSD Overriding Ao Right ventricular hypertrophy (see after birth) |
|
|
4 Chamber view normal in >95% of cases
|
Tetralogy of Fallot
|
|
|
Tetralogy of Fallot OFT imaging key in diagnosis
|
Ascending Ao overrides perimembranous VSD
Pulmonary artery narrow |
|
|
Most Common Cyanotic congenital heart disease
|
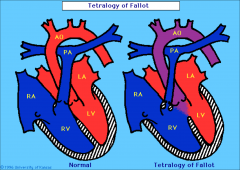
Tetralogy of Fallot
|
|
|
Tetralogy of Fallot Prognosis dependent on aneuploidy
|
Chromosomal abnormality in 45% of cases
Isolated = excellent long term prognosis with repair >94% survival Prognosis worse if there is absence of the pulmonary valve 32% mortality at 4 years |
|
|
Transposition of Great Arteries
|
Ao and PA are switched
Ao comes from right ventricle PA comes from left ventricle |
|
|
OFT’s do not criss cross – instead they run parallel to each other
|
Transposition of Great Arteries
|
|
|
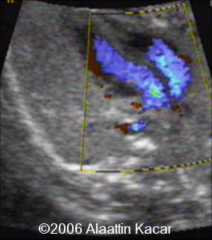
DA and Foramen Ovale = the only communication between right and left side of fetal circulation
These close at birth = BLUE BABY Surgery required within 1st week of life |
Transposition of Great Arteries
|
|
|
Truncus Arteriosis
|
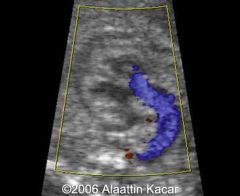
Single vessel (truncus) arising from the heart
Gives rise to Ao and PA Single tuncal valve with 1-6 cusps May cause stenosis +/- regurg |
|
|
40% of liveborn infants with truncus 22q11 deletion
Prognosis dependent on associated abnormalities Outcome worse with interruption of Ao Arch |
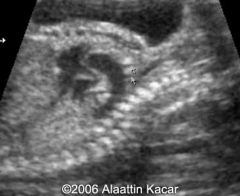
Truncus Arteriosis
|
|
|
Hypertrophic Cardiomyopathy
|
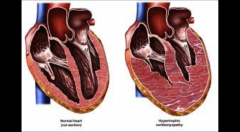
Primary disorder of cardiac muscle
Thickened but non dilated left ventricle |
|
|
causes of Hypertrophic Cardiomyopathy
|
Familial
50% have a genetic mutation in chromosome 1, 14, or 15 Non-familial: Noonan Syndrome, other chromosomal abnormalities Other causes: Diabetes Mellitus 50% type 1 25% type 2 Rare in gestational DM Metabolic Causes Fetal renal disease TTTS |
|
|
Hypertrophic Cardiomyopathy mortality rate
|
High mortality rate – 52%
|
|
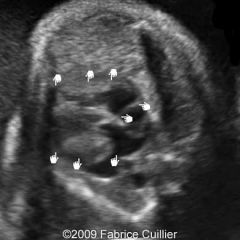
Hypertrophy of myocardium
Asymmetric in distribution Septum most often involved May be confined to apex or free wall May be symmetric (concentric hypertrophy) +/- cardiomegaly |
Hypertrophic Cardiomyopathy
|
|
|
Myocardial disease not usually associated with structural or pericardial malformations
Dilated Heart with decreased systolic function Final common pathway for diverse disease process that leads to heart failure |
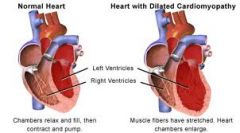
Dilated Cardiomyopathy
|
|
|
Dilated Cardiomyopathy Overall mortality
|
rate 82%
57% IUFD, remainder in neonatal period Hydrops poor prognostic sign Post natal options are few Survivors may require transplant |
|
|
Dilated Cardiomyopathy Imaging
|
Cardiomegaly
Poor myocardial contractility Myocardium thin +/- hydrops Pulsed Doppler: Reserved flow in: IVC DV Pulsatile UV Atrioventricular regurg color and pulsed |
|
|
Ectopic or extra beats which can arise anywhere (atria or ventricle) within myocardium to produce an irregular rhythm
|
Irregular Rhythm
There can be one, many or blocked beats |
|
|
Irregular Rhythm
|
1-2% pregnancies will have an arrhythmia
PAC/PVC account for 90% Usually requires no treatment Most resolve by by delivery – very rare to cause problems in neonate Frequent PAC’s 2-5% risk developing SVT Reduction in maternal caffeine, alcohol, nicotine suggested Periodic monitoring for tachycardia |
|
|
Irregular Rhythm Imaging
|
M-Mode
Curser needs to be placed over atria + ventricle PAC: Early atrial contraction Compensatory pause of left ventricle Normal rhythm resumes |
|
|
Tachyarrhythmia
|
Supraventricular tachycardia (SVT): any tachycardia with an origin above ventricles
Rates typically > 200 |
|
|
with sustained tachyarrhythmia
|
Hydrops develops 50-75%
Harder to treat |
|
|
Tachyarrhythmia Imaging
|
Sustained heart rate > 200
M-mode through atrium + ventricle |
|
|
Atrioventricular Block
|
Transmission of electrical impulses from atria to vents is blocked
|
|
|
Atrioventricular Block May be caused by
|
immature conducting system
Absense of connection to the AV node Abnormal anatomic position of AV node Maternal Lupus increased risk |
|
|
Atrioventricular Block diagnosis
|
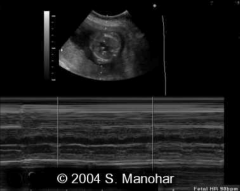
Diagnosed with M-Mode through Atria and vent
2nd degree heart block most commonly seen 2:1 (2 atrial beats to every 1 ventricular beat) |

