![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
75 Cards in this Set
- Front
- Back
|
What is the definition for a transition metal? |
A d-block element that forms an ion with an incomplete d-sub shell |
|
|
Why are Zinc and Scandium not transition metals? |
They do not form ions with incomplete d - sub shells. Sc only forms Sc 3+ in which all d-orbitals are empty. Zn only forms Zn 2+ in which all d-orbitals are full. |
|
|
How do electrons fill orbitals and why? |
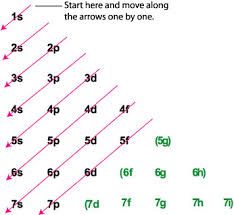
4s fills before 3d because it occupies a lower energy level. |
|
|
How do electrons fill orbitals in a) Chromium b) Copper |
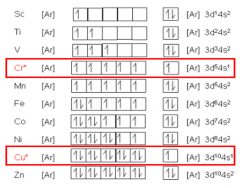
|
|
|
How do transition metals normally form ions? |
By losing electrons to form positive ions |
|
|
Where are electrons lost from first? Why? |
Electrons are lost from the 4s orbital first because when occupies, it has a higher energy level than the 3d orbitals. |
|
|
Describe the normal metallic properties of transition metals |
- Shiny - Dense - High melting and boiling points - When solid, transition metals exist as giant metallic lattices in which electrons are delocalised and move to conduct electricity |
|
|
Outline four uses of transition metals |
1) Nickel is alloyed with Copper for 'silver' coins 2) Titanium is a component for joint replacement parts - for example, the ball and socket joint for hips 3) Iron is cast to make telephone boxes and post boxes 4) Iron is alloyed for use in the construction of buildings and bridges |
|
|
What do transition metals form? |
Compounds in which the transition metal has different oxidation states |
|
|
What do transition metal compounds form? |
Coloured solutions when dissolved in water that frequently catalyse chemical reactions. |
|
|
Why are 4s electrons lost first? |
Because they occupy the highest energy level when filled, but because the energy levels are so close, 3d electrons can also be lost when an atom forms a stable ion. |
|
|
What do strong oxidising agents contain? |
A transition element in its highest oxidation state |
|
|
Give two examples of oxidising agents |
1) Manganese forms potassium permanganate - KMnO4 - a purple solid used as an oxidising agent in redox titrations 7+
2) Chromium forms potassium dichromate - Kr2Cr2O7, an orange crystalline solid that acts as an oxidising agent in the preparation of aldehydes and ketones from alcohols. 6+ |
|
|
Define 'catalyst' |
A substance that increases the rate of a chemical reaction by providing an alternative route for the reaction with a lower activation energy |
|
|
What are the two ways that transition metals can act as catalysts? |
1) By providing a surface for the reaction to occur - Reactants adsorb to the surface of a metal and are held in place while the reaction occurs. After the reaction has occurred, the products are desorbed and the metal remains unchanged. 2) Intermediates - transition metal ions have the ability to change oxidation state by gaining or losing electrons. They then bind to reactants, forming intermediates as part of a chemical pathway with lower activation energy. |
|
|
What are the four processes that are catalysed by transition metals you need to know of? |
1) Haber Process 2) Contact Process 3) Hydrogenation of Alkenes 4) Decomposition of Hydrogen Peroxide, H2O2 |
|
|
Outline the Haber process. Include: - the equation - the catalyst - what the catalyst does - what the product is used for |
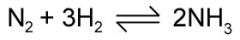
- (g) + (g) -> (g) Iron metal catalyst lowers the temperature and increases the reaction rate. -Ammonia is used for fertilisers.
|
|
|
Outline the Contact process. Include: - the equation - the catalyst - what the catalyst does - what the product is used for |
-Vanadium (V) oxide catalyst - Vanadium is in it's +5 oxidation state - SO3 is used in the manufacture of H2SO4 which is then used for fertilisers, detergents, adhesives and explosives. |
|
|
Outline the hydrogenation of alkenes. Include: - the equation - the catalyst - what the catalyst does - what the product is used for |
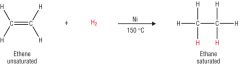
-Nickel catalysts lowers the temperature and pressure required. - Used to make spreadable margarines. |
|
|
Outline the decomposition of hydrogen peroxide. Include: - the equation - the catalyst - what the catalyst does - what the product is used for |

- MnO2 catalyst (Mn 4+) - Increases the rate - Used in the preparation of oxygen gas. |
|
|
Define 'precipitation reaction' |
A reaction in which soluble ions in separate solutions are mixed together to form an insoluble compound that settles out of the mixture as a solid. |
|
|
What is the generalised precipitation reaction of transition metal ions? |
Transition metal ions (aq) + OH- (aq) ---> coloured ppt. |
|
|
What four transition metal ion precipitation reactions do you need to know? |
Cu 2+ Co 2+ Fe 2+ Fe 3+ |
|
|
Outline the precipitation reaction of Cu 2+ and the colour changes that occur. |
Cu2+ (aq) +2OH- (aq) -> Cu(OH)2 (s)
Pale blue solution -> Pale blue ppt. |
|
|
Outline the precipitation reaction of Co 2+ and the colour changes that occur. |
Co2+ (aq) +2OH- (aq) -> Co(OH)2 (s) Pink solution -> blue ppt -> beige in air |
|
|
Outline the precipitation reaction of Fe 2+ and the colour changes that occur. |
Fe2+ (aq) +2OH- (aq) -> Fe(OH)2 (s)
Pale green solution -> Green ppt -> Rusty brown on surface in the presence of air |
|
|
Outline the precipitation reaction of Fe 3+ and the colour changes that occur. |
Fe3+ (aq) +3OH- (aq) -> Fe(OH)3 (s)
Pale yellow solution -> Rusty brown ppt. |
|
|
Define 'complex ion' |
A transition metal bonded to one or more ligands by coordinate bonds |
|
|
Define 'ligand' |
A molecule or ion that donates a pair of electrons to the central metal ion to form a coordinate bond |
|
|
Define 'coordinate bond' |
A bond in which one of the bonding atoms provides both the electrons in the shared pair |
|
|
What do transition metal ions form in solution? |
Complex ions. |
|
|
Annotate [Cu(H2O)6]2+ showing what each part means. |
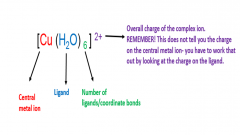
|
|
|
List all the ligands you have to know and their charges. |
Water :OH2 0 Ammonia :NH3 0 Chloride :Cl- -1 Cyanide :CN- -1 Thiocyanate :SCN- -1 Hydroxide :OH- -1
|
|
|
Define Monodentate |
A ligand that donates one pair of electrons to the central metal ion to form one coordinate bond. |
|
|
Describe the most common shape of complex ions - give one example. |
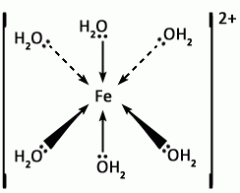
The most common complex ion shape is octahedral, with 6 coordinate bonds, with all bond angles 90°. There are 4 ligands in the same plan, and one above and below this plane.
|
|
|
Describe the two other possible shapes of complex ions |
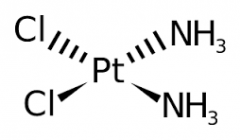
1) Square Planar - four coordinate bonds
2) Tetrahedral - four coordinate bonds
|
|
|
Define 'stereoisomer' |
Species with the same structural formula but with a different arrangement of atoms in space |
|
|
What two types of stereoisomerism do complex ions exhibit? |
Cis-trans Optical
|
|
|
Describe how cis-trans isomerism occurs in octahedral complex ions |
Cis trans isomerism occurs in octahedral complex ions with two types of ligands: four of one and 2 of the other. |
|
|
Outline how cis-trans isomerism occurs in [Co(NH3)4Cl2]+ |
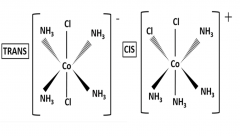
It was observed that Co3+ complex ions formed salts with different colours: this is because of cis-trans isomerism.
In the CIS isomer, the two Cl- ligands are at 90° to one another In the TRANS isomer, the two Cl- ligands are at 180° to one another |
|
|
Outline how cis trans isomerism occurs in four-coordinate complexes. |
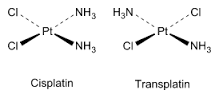
The complex must have two different type of ligands - two of each. |
|
|
Define 'bidentate' |
A ligand that can donate two pairs of electrons to the central metal ion to form two coordinate bonds |
|
|
What is the most common bidentate ligand? |
Ethane -1,2-diamine, NH2CH2CH2NH2, en. Each nitrogen can donate an electron pair. |
|
|
What type of complexes can bidentate ligands form? Give an example. |
![Octahedral.
Example - [Ni(en)3]2+](https://images.cram.com/images/upload-flashcards/04/43/00/9044300_m.jpg)
Octahedral. Example - [Ni(en)3]2+
|
|
|
Octahedral complexes containing bidentate ligands show cis-trans isomerism. Give an example. |
![[Cu(C2O4)2(H2O)2]-](https://images.cram.com/images/upload-flashcards/04/43/21/9044321_m.jpg)
[Cu(C2O4)2(H2O)2]-
|
|
|
What is a hexadentate ligand? |
A ligand that has 6 lone pairs of electrons, each forming a coordinate bond to the central metal ion. |
|
|
Give an example of a hexadentate ligand and its function. |
ETDA - ethylenediaminetetraaceticacid
It is used to bind metal ions. It is known as a chelating agent - it reduces the concentration of metal ions in solution by binding them in a complex.
|
|
|
What are the requirements for optical isomerism in complex ions? |
1) A complex with three molecules or ions of a bidentate ligand - [Ni(en)3]2+
2) A compelx with two molecules or ions of a bidentate ligand and two of a monodentate ligand - [Co(en)2Cl2]
3) A complex with one molecule or ion of a hexadentate ligand - [Cu(ETDA)]2+
|
|
|
Define 'optical isomer' |
Non-superimposable mirror images of one another |
|
|
How do optical isomers differ? |
They rotate plane-polarised light in opposite directions. |
|
|
Define 'Ligand Substitution' |
A reaction in which one ligand in a complex ion is replaced with another ligand |
|
|
What are the three ligand substitution reactions you need to know? |
1) Cu 2+ ions (aq) + NH3 2) Cu 2+ ions (aq) + HCl (conc) 3) Co 2+ ions (aq) + HCl (conc) |
|
|
Write the equations for a) Cu 2+ ions (aq) + NH3 b) Cu 2+ ions (aq) + HCl (conc) c) Co 2+ ions (aq) + HCl (conc) |
a) [Cu(H2O)6] 2+ (aq) + 4NH3 (aq) -> [Cu(NH3)4 (H2O)2 ]2+ + 4H2O(l) b) [Cu(H2O)6] 2+ (aq) + 4Cl¯(aq) ——> [CuCl4] 2- (aq) + 6H2O(l) c) [Co(H2O)6] 2+ (aq) + 4Cl¯(aq) ——> [CoCl4] 2- (aq) + 6H2O(l) |
|
|
Outline the colour changes and nature of the products for a) Cu 2+ ions (aq) + NH3 b) Cu 2+ ions (aq) + HCl (conc) c) Co 2+ ions (aq) + HCl (conc) |
a) (Cu 2+ + NH3) At the start: Pale blue solution After a small amount of ammonia: Pale blue ppt as the intermediate Cu(OH)2 forms After excess of ammonia: Ppt dissolves, deep blue solution forms
Product ( [Cu(NH3)4(H2O)2]2+ ) is DISTORTED OCTAHEDRAL due to the differences in bond lengths: C-O is longer than C-N
b) (Cu 2+ + HCl) At the start: Pale blue solution Initially forms: Green solution Finally forms: Yellow solution Chloride ligands are larger than H2O ligands so less can fit around the central ion: it forms a tetrahedral compound.
c) (Co2+ + HCl) At the start: pink solution End: blue solution Tetrahedral. |
|
|
What is Haemoglobin? |
A protein with four polypeptide chains and a haem group with an Fe 2+ ion at the centre, which oxygen can bind to. When oxygen is bound, the haem group appears red in colour. |
|
|
Describe the transport? ish of haemoglobin round the body. |
As haemoglobin travels round the body it picks up O2 from the lungs and transports it to cells where it is released. Red blood cells can also pick up CO2 as a waste product from cells, which is then transported to the lungs where it is released. |
|
|
Draw and describe the structure of haemoglobin. |
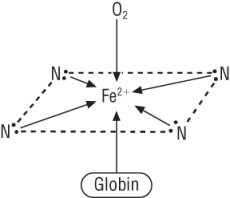
- 4 coordinate bonds from Nitrogen in the haem structure - 1 coordinate bond from the globin protein - 1 coordinate bond from O2 |
|
|
Describe the action of Carbon Monoxide |
Carbon monoxide can bind to haemoglobin at the same binding site as Oxygen. But, carbon monoxide will bind more strongly to haemoglobin than oxygen, so if both are present, carbon monoxide will bind irreversibly and less haemoglobin will be left to pick up oxygen. This means tissues will be starved of oxygen and is an example of ligand substitution. |
|
|
How is carbon monoxide formed? Describe its properties. Describe its effects. |
Carbon monoxide is formed by incomplete combustion of carbon-containing fuels. It is an odourless, colourless gas which is hard to detect (so we have sensors). At low levels, it can cause headaches, nausea and potential suffocation. Tobacco can also release carbon monoxide. |
|
|
What is Kstab? |
An equilibrium constant for the equilibrium that exists between a transition metal ion surrounded by water ligands and the complex that is formed when the same ion has undergone ligand substitution. Kstab = [Products] / [Reactants] ( raised to the power of their balancing numbers)
Water is left out because it remains virtually constant. |
|
|
What does a high value of Kstab indicate? |
The equilibrium lies to the right. The complex ion is more stable. |
|
|
Define 'oxidation' |
The loss of electrons or increase in oxidation number |
|
|
Define 'reduction' |
The gain of electrons or the decrease in oxidation number |
|
|
Outline and explain the oxidation of Fe 2+ ions. |
Fe 2+ (aq) ----> Fe 3+ (aq) + e- Fe 2+ ions are easily oxidised in the presence of air or when in contact with an oxidising agent. |
|
|
Outline and explain the reduction of Mn 7+ |
MnO4 - is a strong oxidising agent. In acidic solution MnO4- is reduced to Mn 2+:
MnO4- (aq) + 8H+ (aq) + 5e- ----> Mn2+ (aq) + 4H2O (l)
|
|
|
What else is MnO4- used for? |
MnO4- is commonly used to oxidise solutions containing Fe 2+ ions.
MnO4- (aq) + 8H+ (aq) + 5Fe2+ (aq) ----> 5Fe3+ (aq) + 4H2O(l) + Mn2+ (aq) |
|
|
What do acid-base reactions involve? |
The transfer of protons from the acid to the base, forming a salt and water. An indicator is used to establish the end-point. |
|
|
What do redox titrations involve? |
The transfer of electrons from on species to another. An oxidising agent can be titrated against a reducing agent. An indicator can be used, but sometimes there is already a colour change. |
|
|
Give an example of a redox titration colour change that naturally occurs. |
MnO4- (aq) ------------------> Mn2+ (aq) Deep purple colour of MnO4- masks any other colours present. Mn2+ appears almost colourless: these ions are normally pale pink but the solution is very dilute and the ions are in such a small concentration, that the solution appears colourless. |
|
|
Describe the redox titration between MnO4- and Fe2+ ions. |
- MnO4- can be used to oxidise Fe 2+ ions. - H2SO4 is used to acidify the solution - HCl is NOT used because it will react with the MnO4- and release chlorine gas. - Potassium manganate (VII) is put in the burrette and the iron (II) solution in the conical flask. - As manganate is added to the iron (II) solution, it is decolourised - The end point is established upon the first permanent pink colour - showing an excess of MnO4- ions. |
|
|
Write the reaction between iodine and thiosulfate and describe what happens. |
2S2O3 2- (aq) + I2 (aq) ----> 2I- (aq) + S4O62- (aq)
I2 is reduced to I- ions by aqueous sodium thiosulfate. Aqueous sodium thiosulfate forms the tetrathionate ion, S4O6 2-.
|
|
|
How can the concentration of idiodine be determined? |
By titration with a solution of sodium thiosulfate of known concentration. This titration can be used to determine the concentration of a solution of an oxidising agent (Ex. ClO-, Cu 2+, and Cr2O7 2-) that reacts with iodide ions, I- to produce iodine, I2.
|
|
|
What is the method for analysing an oxidising agent? |
Iodide ions, I-, are added to the oxidising agent. A redox reaction occurs, producing Iodine, I2. This Iodine is titrated against a sodium thiosulfate solution of known concentration. From the results, you can calculate the amount of idoine and thus the concentration of the oxidising agent.
|
|
|
Write the equation for the redox reaction between Cu 2+ and Iodide ions and explain what's going on. |
2Cu 2+ (aq) + 4I- (aq) ----> 2CuI (s) + I2 (aq) Iodide ions are oxidised to iodine: 2I- ---> I2 + 2e- Cu 2+ ions are reduced to Cu + Cu2+ + e- -----> Cu +
This reaction produces a light brown/yellow solution and a white precipitate of CuI (s). The precipitate, however, appears light brown due to the iodine in the solution.
A blue/black colour forms, and disappears sharply at the end point because all the iodine has reacted. The results can then be used to determine the concentration of iodine in the titration mixture and then the concentration of Cu 2+ ions in the original solution.
|
|
|
What can this method be used to do? |
Estimate the concentration of Cu 2+ ions in an unknown solution or the percentage of copper in alloys such as brass and bronze. The alloy is first reacted with Nitric acid. Brass and bronze can both be reacted to produce a solution containing Cu 2+ ions. This then can be reacted with potassium iodide solution. The idoine that forms is then titrated against sodium thiosulfate. |

