![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
5 Cards in this Set
- Front
- Back
|
Nucleophilic Aromatic Substitution |
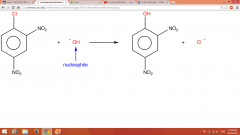
Performed in base (NaOH + H2O) @ RT |
|
|
Elimination-Addition |
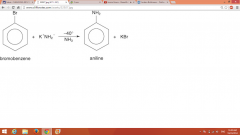
Intermediate with triple bond = benzyne (source of NH2 is KNH2; uber strong base) |
|
|
Oxidation of Phenols |

Product = quinone; could also use Na2Cr2O7 in H2SO4/H2O or O3 |
|
|
EAS of Phenols |
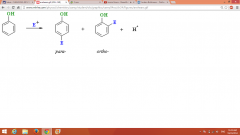
E+ could be Br2 in CCl4 (or in CS2) |
|
|
Williamson Ether Synthesis |
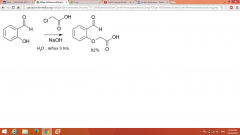
Leaving group allows for the creation of carbon-carbon bonds to form an ether |

