![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
33 Cards in this Set
- Front
- Back
|
Ideal gas law assumes |
No IMF atoms/molecules occupy no space |
|
|
IMF real gases feel as a function of seperation |
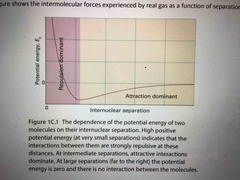
Back (Definition) |
|
|
Ideal gas limiting law |
Works best if p—-> 0 |
|
|
Z is compression factor |
For ideal gas Z = 0 Attractive forces Z < 1 Repulsive forces Z > 1 |
|
|
Ideal gas works best when? |
Between 200-10,000 k and below 10 atm |
|
|
Vm=? |
V/n |
|
|
When do atoms feel IMF |
High pressures and low temps |
|
|
When does ideal gas work best |
Larger seperation |
|
|
Z (compression factor) |
Z=(pVm/RT)=(pv/nRT) |
|
|
Viral equation |
-Accounts for multiple atoms pVm=RT(1+ B/Vm+C/Vm^2....) B and C are known at several temps |
|
|
Van der waal equation |
-addresses assumptions of ideal gas law P=(nRT/(V-nb))-a (n^2/V^2) A accounts for IMF and B accounts for volume atoms occupy |
|
|
System |
All materials involved in process being studied |
|
|
Surroundings |
Everything else in universe not the system |
|
|
Open system |
Matter and energy can move between system and surroundings |
|
|
Closed system |
Matter cannot move between system and surroundings but energy can |
|
|
Isolated system |
System that cannot exchange matter or energy between system and surroundings |
|
|
Adiabatic |
Boundary that does not transfer heat |
|
|
Diathermal |
Boundary that does transfer heat |
|
|
Energy |
-ability to do work -transfered as heat or work |
|
|
Work |
Energy transfer by coordinated movement of matter |
|
|
Heat |
Increase in random motion of matter |
|
|
U |
-internal energy,total energy of system Delta u=q+w State function |
|
|
q and w |
Path functions. Describe how process is carried out |
|
|
Work against contestant external pressure |
W=-Pex(Vf-Vi) |
|
|
Free expansion in vacuum. |
Pex =0 W=0 |
|
|
Isothermal, reversible ideal gas |
w=-nRT ln (Vf/Vi) |
|
|
Energy transfer quantified by heat capacity |
Constant volume Cv Constant pressure Cp |
|
|
Thermochemistry |
Study of heat flow during physical and chemical changes |
|
|
Standard state |
Specified temp. Is the pure form at 1 bar |
|
|
Constant volume |
W=0 |
|
|
Adiabatic |
q=0 |
|
|
Isothermal ideal gas |
Delta U = 0 |
|
|
Enthalpy |
H=U+pV |

