![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
Outline the basic structure and function of haemoglobin and myoglobin.
|
• Both are oxygen-binding proteins, overcoming the problem of low oxygen solubility in blood plasma.
• Haemoglobin: tetrameric haem protein found in erythrocytes, where it is responsible for binding oxygen in the lung and transporting it throughout the body and facilitating the return of carbon dioxide from the tissues. • Haemoglobin is a monomeric haem protein found mainly in muscles. It rapidly delivers oxygen to hypoxic tissues in respiring tissues. |
|
|
What is co-operativity and how is it shown by haemoglobin, but not myoglobin?
|
• Affinity of macromolecule for ligand changes with amount of ligand already bound.
• Shown by haemoglobin - sigmoidal oxygen dissociation curve, not my myoglobin - rectangular hyperbola dissociation curve. |
|
|
What is the Bohr effect, and what causes it?
|
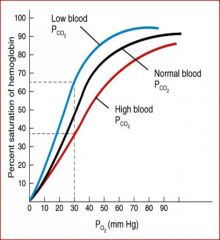
• Increase in pH/decrease in carbon dioxide concentration increases haemoglobin's affinity for oxygen and vice versa.
• About 15% of CO2 produced is carried by Hb. The rest is dissolved, forming carbonic acid/H2CO3 that dissociates to yield H+ - catalysed by carbonic anhydrase. Hb carries 40% of the protons produced. • Protonated His in ß-subunit becomes hydrogen bonded to Asp residue, stabilises T-state/de-oxygenated form and conferring high pKa value. At the lungs Hb shift back to R-state normalises bonding and lowers pKa. • The formation of carbamino-Hb bonds in the N-termini also stabilises the T-state due to the presence of salt bridges, hence contributing to Bohr effect. |
|
|
What is the BPG effect and its effect on oxygen binding?
|
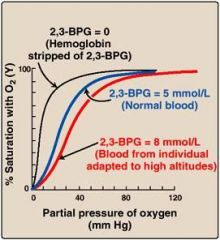
• BPG, D-2,3-bisphosphoglycerate lowers the affinity of blood for oxygen when compared to pure haemoglobin.
• BPG binds to a pocket in the centre of the Hb tetramer when in the T-state, stabilising it - this is less effective in foetal Hb (meaning it has a higher affinity for oxygen). |
|
|
What is the structure of haemoglobin?
|
• A dimer of heterodimers, two alpha subunits and two beta subunits/two alpha-beta dimers.
• Haem (a prosthetic porphyrin ring) is incorporated during protein synthesis, stabilised by hydrophobic residues on the interior that protects the Fe(II) from oxidation - which leads to malfunction. • 26.3% sequence identity between alpha and beta chain. |
|
|
How does the proportion of different types of haemoglobin change during foetal development?
|
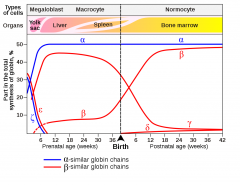
• 3 types of Hb:
-HbE: Gower I (ζ2ε2), Gower II (α2ε2), Portland (ζ2γ2) -HbF: α2γ2 -HbA: α2ß2 |
|
|
Give more information on the binding of the prosthetic haem group.
|
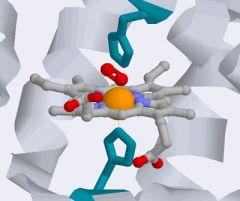
• 8 alpha helices are connected by turns and loops in Globin fold, with haem binding between E and F. There are histidine residues near the haem iron atom.
• The distal histidine (F7) stabilises the oxygen-bound state of Hb and reduces the affinity of CO to haem 200 fold, when compared to 'free' haem. |
|
|
What are the oxygen-induced structural changes in haemoglobin?
|
• Induces movement of Fe(II) towards the haem plane - by 0.4Å.
• Causes movement of F helix. • Leads to conformation shape change that facilitates allosteric transition from the T state to R state. |
|
|
Sickle cell disease
|
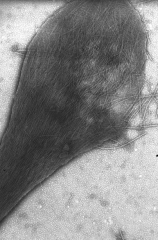
• Genetic condition resulting from a single nucleotide polymorphism that converts glutamate (-ve) to valine (non-polar) in the ß chain. This causes hydrophobic patches.
• The oxygenated molecules are soluble but when de-oxygenation occurs the conformation of HbS differs considerably from HbA and it can aggregate to form insoluble fibres - in a 'sickle crisis'. These fibres deform the RBCs into sickle-shapes. |
|
|
What are some other clinical considerations?
|
• Low Fe(II) intake in iron may lead to anaemia.
• Inherited defects in synthesis of haem groups can give rise to a variety of prophyria diseases. • Reduced synthesis of alpha or beta chains can result in thalassemia. • Diabetic patients will have a heightened amount of glycosylated haemoglobin in their red cells, HbA1C. Easily measured in blood sample by HPLC. |

