![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
75 Cards in this Set
- Front
- Back
|
What is the most common bone disease in humans?
|
Osteoporosis
|
|
|
What are some of the functions of Ca2+?
|
- Second messenger
- Cofactor for enzymes (e.g., blood coagulation) - Stabilizes proteins - Muscle and cardiac cell contraction - Vascular patency - Structural component of teeth and bone - Nerve conduction |
|
|
What controls blood Ca2+ levels?
|
* Vitamin D
* Parathyroid Hormone (PTH) * Calcitonin * Estrogens * Androgens - Insulin-like growth factors I and II - Transforming Growth Factor-beta - Interleukins - Prostaglandins - Tumor Growth Factor family proteins |
|
|
What is the main result of Osteoporosis? Why?
|
Low bone density - loss of bone tissue by excessive degradation of bone by osteoclasts / excessive bone remodeling --> increased fracture risk
|
|
|
How much does the bone density need to decline to meet the requirement for osteoporosis?
|
2.5 standard deviations below the mean for young healthy adults of the same gender
|
|
|
Which gender is more likely to have Osteoporosis? How many?
|
- Women (especially postmenopausal) > men
- Women: 8 million - Men: 2 million |
|
|
What is necessary for Vitamin D to control Ca2+ levels in bone and blood?
|
Vitamin D must be metabolized to active form: 1,25-dihydroxy vitamin D (calcitrol)
|
|
|
How does 1,25-dihydroxy Vitamin D (Calcitrol) affect Ca2+ and phosphate?
|
It induces synthesis of proteins that bind calcium and phosphate in the cells of intestine, bone, muscle, etc.
|
|
|
How does the amount of Ca2+ and Vit. D required from diet change with age?
|
Increases with age
|
|
|
What are the steps of bone remodeling occur?
|
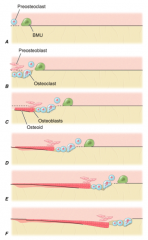
1. Exposure of BM collagen
2. Fusion of osteoclasts; resorption of cavity by osteoclasts and mononuclear cells; proliferation of preosteoblasts 3. Osteoblasts align and start forming osteoid 4. Continued osteoblast deposition of osteoid and mineralization 5. Flattening of osteoblasts 6. Osteoblasts turn into lining cells; bone remodeling complete |
|
|
How do Preosteoclasts get activated?
|
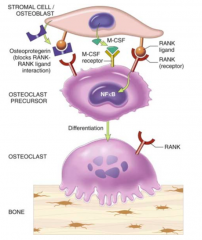
- Interaction of Preosteoclasts w/ Osteoblasts through RANK-Ligand (on blasts) and RANK receptors (on clasts)
- Osteoblasts synthesize CSF-M which stimulates activation |
|
|
How do you regulate the activation of Preosteoclasts to prevent too much bone resorption?
|
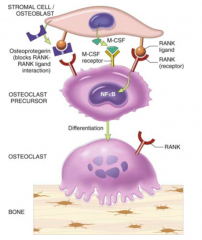
Osteoprotegenin (OGP) on osteoblasts inhibits activation of Preosteoclasts by acting as a decoy receptor for RANK-ligand on osteoclasts
|
|
|
During bone remodeling, what else is remodeled?
|
ECM surrounding bone (collagens / proteoglycans)
|
|
|
Is there a genetic component to Osteoporosis?
|
Yes, but the genes involves have not been identified
|
|
|
What are good dietary sources of Ca2+?
|
- Milk and milk products (yogurt cheese, ice cream)
- Dark green leafy vegetable (broccoli, bok choy, collards, kale, mustard turnip greens, soybeans) - Fish canned with soft bones - Fortified orange juice, tofu and breads |
|
|
How much Ca2+ do the following groups require?
- 4-8 - 9-18 - 19-70 - Pregnant/lactating - Post-menopausal women |
- 4-8: 1000 mg
- 9-18: 1300 mg - 19-70: 1000 mg - Pregnant/lactating: 1300-1500 mg - Post-menopausal women: 1200-1500 mg |
|
|
What minimum amount of Ca2+ is necessary for adults for fracture prevention?
|
1000 mg of Ca2+
|
|
|
What is the highest amount of Ca2+ that is safe to ingest? Why is this important?
|
2000-2500 mg / day - can impair kidney function and inhibit reabsorption of other minerals (iron, zinc, magnesium, phosphorus)
|
|
|
How much Ca2+ can be absorbed by the intestine at a time?
|
500 mg (so need to spread out the Ca2+ intake throughout the day)
|
|
|
What does Vitamin D bind to, to regulate gene expression?
|
Vitamin D Nuclear Receptor (VDR)
|
|
|
What are the forms of dietary Vitamin D?
|
- Plants: Vitamin D2 - ergocalciferol (extra double bond at C22)
- Animal products: Vitamin D3 - cholecalciferol |
|
|
What are good sources of vitamin D?
|
- Fish
- Eggs - Fortified cheese, butter, margarine, milk, cereals |
|
|
What is the recommended daily intake for Vitamin D?
- <50 - 50-70 - >70 |
- <50: 200 IU
- 50-70: 400 IU - >70: 600 IU |
|
|
How is Vitamin D taken up from the diet?
|
In chylomicrons (similar to that of lipids)
|
|
|
How can Vitamin D be obtained if not through the diet?
|
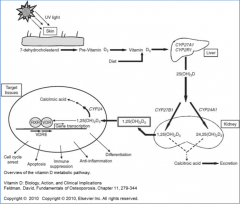
- Synthesized from 7-dehydrocholesterol (intermediate in cholesterol pathway)
- Converted in skin in two steps, first in response to UV light, second spontaneously |
|
|
How much Vitamin D is synthesized by citizens of Wisconsin from November to February?
|
Essentially no cutaneous Vitamin D
|
|
|
Vitamin D (D2 or D3 from diet, or D3 from skin) is further converted how?
|
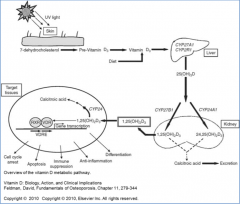
- Sent to liver where it is hydroxylated at 25 position by P450 monooxygenase to 25-OH-D3 (calcifediol)
- Transported to kidney where it hydroxylated at 1 position by another P450 monooxygenase to 1,25-(OH)2-D3 (calcitriol) |
|
|
How is activation of Vitamin D regulated?
|
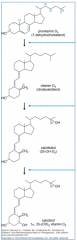
- Hydroxylation at 25 position is unregulated in liver
- Hydroxylation at 1 position is stimulated in kidney by PTH, low phosphate, low calcium, estrogen, and prolactin |
|
|
What can inhibit the hydroxylation of 25-OH-D3 in kidney to 1,25-(OH)2-D3?
|
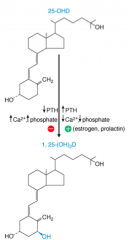
- Low PTH
- High Ca2+ - High Phopshate - Product: 1,25-(OH)2-D (negative feedback) |
|
|
What proteins that are stimulated by Vitamin D, bind minerals?
|
- Calbindin - in intestine - Ca2+ and phosphorus
- Osteocalcin - in bone - Ca2+ and phosphorus - Troponin C - in muscle - Ca2+ |
|
|
Besides proteins that bind Ca2+ and phosphorus, what other genes does Vitamin D regulate transcription of?
|
Genes that regulate cell proliferation, apoptosis, and differentiation amino acid uptake (regulates immune, endocrine, neurological, and CV functions)
|
|
|
What is the mechanism of Vitamin D regulating gene expression?
|
- 1,25-dihydroxy Vitamin D binds to Vitamin D receptor (VDR)
- Receptor forms heterodimer w/ retinoic acid receptor, RXR bound to 9-cis-retinoic acid - VDR-RXR complex binds to promoters of Vitamin D responsive genes and initiates formation of complexes of proteins that initiate transcription |
|
|
What is Vitamin D metabolized to for excretion?
|
Calcitronic Acid
|
|
|
What can cause Vitamin D deficiency?
|
- Lack of vitamin D in diet
- Lack of sunlight to endogenously make vitamin D from 7-dehydrocholesterol - Deficiency of enzymes required to convert Vitamin D into active 1,25-dihydroxy form (genetic deficiency) - Fat malabsorption syndromes (CF, IBD) can limit absorption of Vitamin D from intestine - Chronic liver disease inhibits ability of liver to convert to active form (1st step) - Chronic renal disease inhibits ability of kidney to convert to active form (2nd step) |
|
|
What is the potential impact of Vitamin D deficiency?
|
- Osteoporosis in adults (mild deficiency)
- Rickets in children (severe deficiency) - Osteomalacia in adults (severe deficiency) |
|
|
What causes Rickets? Who gets it? What are the symptoms? How is it treated?
|
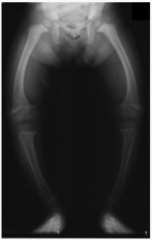
- Extreme Vitamin D deficiency
- Children - Growth retardation w/ growth plate expansion and bowing of legs - Treat w/ vitamin D prior to epiphyseal fusion (reverse effects) |
|
|
What causes Osteomalacia? Who gets it? What are the symptoms? How is it treated?
|
- Extreme Vitamin D deficiency
- Adults - Impaired mineralization of bones; bones are soft, painful, and bendable w/ bowing of weight bearing bones |
|
|
What are the functions of Parathyroid Hormone (PTH) that are related to Ca2+?
|
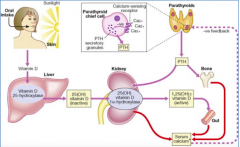
- Regulates serum Ca2+ levels
- Directly regulates release of Ca2+ from bone and reabsorption of Ca2+ by kidney - Stimulates conversion of Vitamin D to final active form in kidney - Indirectly controls Ca2+ uptake by intestinal epithelial cells |
|
|
How does Ca2+ affect PTH?
|
- Inhibits PTH synthesis
- Inhibits PTH release - Modulates degradation of PTH in blood |
|
|
How are the actions of PTH mediated?
|
Binding to PTH receptors on basolateral side of cells causing activation of signaling pathways that stimulate Ca2+ regulation
|
|
|
What are the other, non-Ca2+ related functions of PTH?
|
- Stimulating collagen synthesis
- Regulation of synthesis of DNA, proteins, and phospholipids |
|
|
What is the function of Calcitonin?
|
- Inhibits bone resorption by suppressing osteoclast activity
- Decrease calcium uptake by intestine - Stimulate calcium excretion by kidneys |
|
|
Is Calcitonin a good treatment for Osteoporosis? Why or why not?
|
Yes - it inhibits osteoclast activity (need to be careful for
|
|
|
How does estrogen affect calcium balance / bone? How does progesterone this?
|
- Estrogen w/ and w/o progesterone decrease bone turnover and can induce some increase in bone mass and decreased numbers of fractures
- Estrogens bind nuclear receptors and affect gene expression --> decreased killing of osteocytes and osteoblasts and increased killing of osteoblasts |
|
|
Why is estrogen therapy not used very much for Osteoporosis?
|
Associated w/ increased risk of breast cancer, heart attacks, strokes, and venous thromboembolisms
|
|
|
What is the compound phosphate is in, in the bone?
|
Apatite = Ca5-(PO4)3-X
X= F, Cl, or OH |
|
|
How are blood phosphate levels regulated?
|
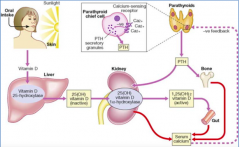
- Dietary levels of phosphate
- 1,25-dihydroxy-Vitamin D (Calcitriol) - increases uptake in intestine, increases resorption from bone, and inhibits renal excretion - Calcitonin - stimulates osteoblasts and excretes phosphate by kidney - PTH - stimulates resorption |
|
|
How do you diagnose Osteoporosis?
|
- DEXA scan (Dual Energy X-ray Absorptiometry)
- T score = compare to young, healthy person matched for race and gender - Z score = age-matched control, same race and gender - A T-score, > 2.5 standard deviations below mean = Osteoporosis |
|
|
What is the greatest cause of osteoporosis?
|
Excessive bone remodeling
|
|
|
What are the risk factors for Osteoporosis?
|
- Lack of exercise
- Chronic RA - Chronic kidney dz - Chronic liver dz - Eating disorders - Chronic corticosteroid use - Hyper-PTH - Family Hx - Amenorrhea for long time - Alcoholism (>3 drinks/day) - Caffeine intake (b/c not drinking milk) - Low body weight - Smoking - Lack of Ca2+ or Vitamin D - Post-menopause women |
|
|
What is the female athlete triad?
|
- Eating disorders (not eating enough calories for the amount of calories they are burning)
- Amenorrhea - Osteoporosis (Z scores < -2 w/o risk factors) |
|
|
Why does the female athlete triad lead to amenorrhea?
|
- Low energy available
- Inhibits GnRH - Decreases LH secretion - Ovarian suppression - decreased estrogen - Functional Hypothalamic Amenorrhea |
|
|
What are the most common sites for DEXA scans?
|
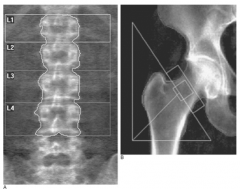
- Lumbar spine
- Hip - Also radius, ulna, calcaneus, and phalanges can be used |
|
|
What do the DEXA score ranges mean?
|
- Normal: < 1.0 S.D. below normal
- Osteopenia: 1.0-2.5 S.D. below normal - Osteoporosis: >2.5 S.D. below normal * Applies to Caucasian Post-menopausal women only * |
|
|
What is the first line of prevention and treatment for osteoporosis?
|
- Healthy lifestyle (exercise and well-balanced diet)
- Adequate calcium and vitamin D intake - Pharmaceuticals that regulate osteoblasts and osteoclasts |
|
|
What is the best exercise for Osteoporosis? How does it help?
|
- Weight bearing exercise (e.g., walking, dancing, cross-country skiing, racquet sports, and weights)
- Prevents loss of bone density - Does not result in much gain of bone density |
|
|
What is the recommended Ca2+ intake for someone with Osteoporosis? Vitamin D?
|
- Ca2+: 1200 mg daily for post-menopausal women
- Vitamin D: 800-1000 IU daily (>1000IU for deficiencies and chronically ill) |
|
|
Besides Osteoporosis, when is Vitamin D supplemented?
|
Treatment of:
- Rickets - Osteomalacia - Hypo-PTH |
|
|
When should bone mass density testing be performed?
|
- Women >65 yo and Men >70 yo, regardless of clinical risk factors
- Younger post-menopausal women, women in menopausal transition, and men 50-70 yo w/ clinical risk factors for fracture - Adults who have a fracture after age 50 yo - Adults w/ RA or taking meds (glucocorticoids >5mg for >3 mo) or associated w/ low bone mass or bone loss - 1-2 years after initiating therapy to reduce fracture risk and every 2 years after |
|
|
When should you start treatment for Osteoporosis?
|
Postmenopausal women and men >50 yo:
- Hip or vertebral fractures - T scores <2.5 at femoral neck, total hip, or lumbar spine by DEXA - Low bone mass (T score 1-2.5 = osteopenia) at femoral neck, total hip, or lumbar spine by DEXA and 10-year hip fracture probability >3% or 10-year major osteoporosis related fracture probability >20% according to Fracture Risk Assessment Tool = FRAX) |
|
|
When should the FRAX (Fracture Risk Assessment Tool be utilized?
|
- Decision to use pharmacological treatment is uncertain
- Men and post-menopausal women age 40-90 that are not on treatment, have low bone mass (T score between 1 and 2.5), no prior hip or vertebral fracture |
|
|
What are the current pharmacological treatments options for Osteoporosis?
|
- Bisphosphanates (Alendronate)
- Calcitonin - Raloxifene (estrogen agonist/antagonist) - Estrogens - Teriparatide (PTH hormone) - Denosumab (RANK-L inhibitor) |
|
|
What are the treatment goals for Osteoporosis?
|
Restore bone growth and prevent fractures
|
|
|
What kind of drug is Alendronate? Mechanism
|
- Bisphosphanate
- Treatment for Osteoporosis and Paget's disease - Analog of pyrophosphate that is incorporated into bone matrix and released slowly as bone is resorbed by osteoclasts - Promotes osteoclast apoptosis and prevents osteoclast anchoring / attachment (via inhibiting farnesyl pyrophosphate synthase) - Remains in bone for months or years until bone is resorbed |
|
|
What are the side effects of Alendronate (bisphosphanate)?
|
- GI disturbances
- Osteonecrosis of jaw (IV) |
|
|
How is Calcitonin delivered as a treatment for Osteoporosis?
|
- Synthetic salmon prep (nasal)
- Synthetic human calcitonin prep - SubQ or IM injection or intranasally |
|
|
What are the side effects of Calcitonin?
|
- Nausea (injected)
- Rhinitis (nasal) |
|
|
What happens after a few days of Calcitonin administration?
|
Patients become refractory - likely d/t receptor downregulation
|
|
|
What kind of drug is Teriparatide? Mechanism?
|
- Synthetic form of PTH
- Anabolic effect on bone - stimulates osteoblast activity and enhances bone formation - Inhibits apoptosis of osteoblasts - Activates bone remodeling through stimulation of IGF-1 and collagen production |
|
|
What is the major use for Teriparatide? Major concern?
|
- Used for osteoporosis in men and women at high risk for fracture
- Black box warning: increase in osteosarcoma in rats (contraindicated in patients predisposed to osteosarcoma) - Also, transient hypercalcemia, nausea |
|
|
When can Estrogen be used for Osteoporosis?
|
Only for Osteoporosis prevention in women with significant ongoing vasomotor symptoms (eg., hot flashes) who are NOT at increased risk for CV disease
|
|
|
What kind of drug is Raloxifene? Mechanism?
|
- Selective estradiol receptor modulator (SERMs)
- Estrogen agonist on bone - Inactive on uterus - Anti-estrogen on breast - Stabilizes and modestly increases bone mass density and has been shown to reduce risk of vertebral compression fracture |
|
|
What are the uses and side effects of Raloxifene?
|
- Prevention and treatment of osteoporosis in post-menopausal women
- Increased thromboembolism and increased vasomotor symptoms (e.g., hot flashes) - Contraindicated in pregnant women, women w/ thromboembolic disorders |
|
|
What kind of drug is Denosumab? Mechanism?
|
- Human monoclonal antibody
- Binds w/ high affinity to RANK-L - Blocks osteoclast formation and activation - Increases BMD and decreases bone turnover |
|
|
What are the uses and side effects for Denosumab?
|
- Treat osteoporosis in men and post-menopausal women at high risk for fractures
- >30% - fatigue, weakness, nausea |

