![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
33 Cards in this Set
- Front
- Back
|
What are the stages of wound healing?
|
1. Hemostasis
2. Inflammation 3. Proliferation 4. Maturation |
|
|
What happens during the first stage of wound healing? What signals mediate this?
|
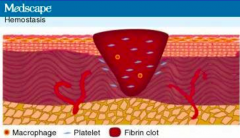
Hemostasis
- Reflexive vasoconstriction (endothelin) - Platelet activation, primary clot formation (bradykinin, serotonin, thromboxane A2) - Release of cytokines, chemokines, and hormones (catecholamines and prostaglandins) |
|
|
What happens during the second stage of wound healing, after hemostasis? What signals mediate this?
|
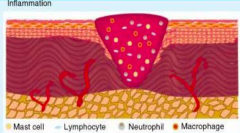
Inflammation
- Vessel dilation, increased vascular permeability (kinins, histamine, prostaglandins, leukotrienes) --> wound edema - Leukocyte (neutrophil and macrophage) recruitment |
|
|
What are the signs of inflammation?
|
- Erythema
- Heat - Edema - Pain |
|
|
What is the purpose of neutrophils during the inflammatory stage of wound healing?
|
Cleanse wound site and release inflammatory mediators - prominent during first 48 hours after injury
|
|
|
What is the purpose of macrophages during the inflammatory stage of wound healing?
|
- Phagocytize debris and bacteria
- Secrete collagenases and cytokines --> proliferation of fibroblasts, smooth muscles, and endothelial cells - Essential during early phase of wound healing |
|
|
What happens during the third stage of wound healing, after inflammation?
|
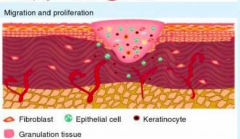
Proliferation
- Fibroblasts, smooth muscle cells, and endothelial cells infiltrate wound and reestablish tissue continuity - Fibroblasts proliferate and synthesize collagen and matrix metalloproteinases - Epithelialization reestablishes external barrier that minimizes fluid loss and bacterial invasion - Epidermal thickening along wound edges - Wound contraction |
|
|
What happens during the fourth stage of wound healing, after proliferation? What signals mediate this?
|
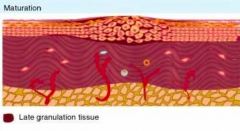
Maturation
- Granulation tissue remodeling and scar formation - Inflammatory cells cleared from scar tissue - Myofibroblasts undergo apoptosis - Collagen undergoes reabsorption to remodel and strengthen wound |
|
|
What is the difference between primary healing and secondary healing?
|
- Primary - uncomplicated healing of non-infected wounds
- Secondary - excessive generation of granulation tissue followed by epithelialization; usually involves infection |
|
|
What are the unique abilities of the keratinocytes in the epidermis?
|
- Regenerate via mitosis
- Repair any defect as long as underlying dermis is not damaged - Minimize trans-epidermal water loss - Barrier to chemical or microbiological attack |
|
|
Any defect of the skin can be repaired if what condition is met?
|
Dermis is not damaged
|
|
|
What are the characteristics of innate cutaneous immunity?
|

- First-line, fast, non-specific mechanism against toxins and microbes
- Stratum corneum (keratinocytes linked by desmosomes) creates anatomical barrier - Chemical mediators (cytokines), complement cascade, leukocytes, and host defense peptides (antimicrobial peptides) all provide intrinsic protection - Phagocytic cells destroy microbes before they can establish an infection |
|
|
What immune cells are part of the innate cutaneous immunity? Functions?
|
- Neutrophils
- Macrophages - Mast cells - Natural killer cells - Upon activation cause tissue injury followed by cytokine release and recruitment of other immune cells to injured site |
|
|
What are the characteristics of adaptive cutaneous immunity?
|
- Acquired response against specific antigens
- Several days to respond (subsequent exposures are faster and more robust) - Stimulation leads to memory - B and T lymphocytes and APCs (Langerhans cells in epidermis) |
|
|
What are the pro-inflammatory cytokines?
|
IL-1, IL-6, TNF-α, IFN-γ
|
|
|
What are the antimicrobial peptides?
|
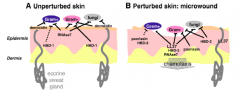
- β-defensins
- Cathelicidin LL-37 - Psoriasin - RNase 7 |
|
|
What kind of cells are characterized by their ability to phagocytize and eliminate pathogens?
|
Macrophages and PMNs
|
|
|
What type of cell is the earliest phagocytic cell to appear during inflammation?
|
PMNs
|
|
|
What type of cell is important in defense against extracellular parasites and involved in hypersensitivity reactions?
|
Eosinophils
|
|
|
What kind of cells are effector cells of immediate hypersensitivity reactions, have a role in eradication of parasites, during acute bacterial infections and in wound healing?
|
Mast Cells
|
|
|
What kind of cells can recognize and kill altered cells, via loss of major MHC1 molecules, which are normally expressed on all nucleated cells?
|
Natural Killer (NK) cells
|
|
|
What kind of cells after stimulation can produce complement components, antimicrobial peptides, cytokines, and chemokines?
|
Keratinocytes
|
|
|
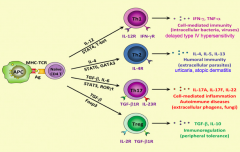
What are the types of mature T cells naive CD4+ T cells can differentiate into?
|
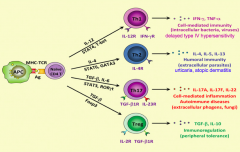
- Th1
- Th2 - Th17 - Treg |
|
|
What signals are necessary to turn a naive CD4+ T cell into a Th1 cell? Function?
|
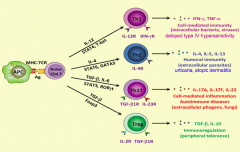
- IL-12
- STAT-1 and STAT-4 (activate master regulator transcription factor TGF-β) - T-bet - Release IFN-γ and TNF-α - Cell-mediated immunity (intracellular bacteria, viruses) and delayed type 4 hypersensitivity |
|
|
What type of T cell is important for cell-mediated immunity (intracellular bacteria, viruses) and delayed type IV hypersensitivity reactions? What mediators lead to this differentiation?
|
- Th1 cell
- Via IL-12, STAT-1, STAT-4, and T-bet |
|
|
What signals are necessary to turn a naive CD4+ T cell into a Th2 cell? Function?
|
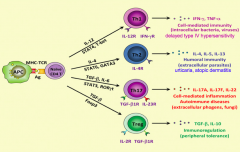
- IL-4
- STAT-6 - GATA-3 - Release IL-4, IL-5, IL-10, and IL-13 - Humoral immunity (extracellular parasites) - Urticaria - Atopic dermatitis |
|
|
What type of T cell is important for humoral immunity (extracellular parasites), urticaria, and atopic dermatitis? What mediators lead to this differentiation?
|
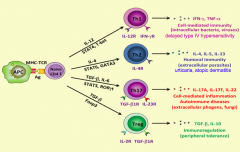
- Th2 cells
- IL-4, STAT-6, GATA-3 |
|
|
What signals are necessary to turn a naive CD4+ T cell into a Th17 cell? Function?
|
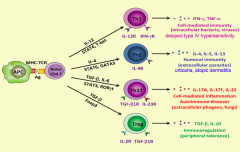
- TGF-β
- IL-6 - STAT-3 - RORγt - Release IL-17A, IL-17F, IL-22 - Cell-mediated inflammation, autoimmune diseases, extracellular phogens, fungi |
|
|
What type of T cell is important for cell-mediated inflammation, autoimmune diseases (extracellular phogens, fungi)? What mediators lead to this differentiation?
|
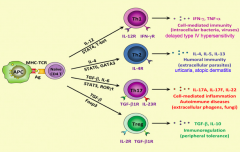
- Th17 cells
- Via TGF-β, IL-6, STAT-3, RORγt |
|
|
What signals are necessary to turn a naive CD4+ T cell into a Treg cell? Function?
|
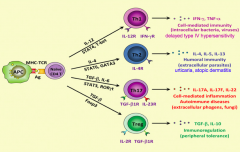
- TGF-β
- Foxp3 - Release TGF-β and IL-10 - Immunoregulation (peripheral tolerance) |
|
|
What type of T cell is important for immunoregulation (peripheral tolerance)? What mediators lead to this differentiation?
|
- Treg cells
- Via TGF-β and Foxp3 |
|
|
How do the Th1 and Th2 responses interact?
|
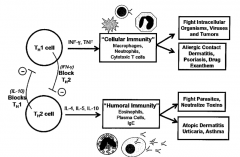
- Both release cytokines that suppress the opposing pathway (IFN-γ inhibits Th2; IL-10 inhibits Th1)
- Negative feedback polarizes the immune response - Can perpetuate disease or impair ability to respond normally - Th1 --> cellular immunity via IFN-γ and TNF-α - Th2 --> humoral immunity via IL-4, IL-5, IL-10, IL-13 |
|
|
Are neutrophils necessary for the early phase of wound healing?
|
- Not necessary (they are just cleansing wound and releasing inflammatory mediators)
- Macrophages on the other hand are very important |

